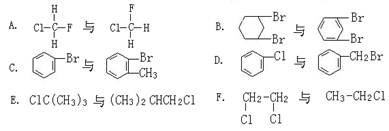
��Ŀ����
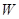 ��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��
��һ���л��ϳ��м��壬�ṹ��ʽΪ��HOOC��CH=CH��CH=CH��COOH��
��1��W�ܷ�����Ӧ�������� ������д��ĸ��ţ�
A��ȡ����Ӧ B��ˮ�ⷴӦ C��������Ӧ D���ӳɷ�Ӧ
��2����֪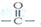 Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�
Ϊƽ����ƽ��ṹ����W����������� ��ԭ����ͬһƽ���ڡ�
��3��������Q��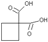 ����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ����
����һ��ҩ���м��壬���ںϳɿ���ҩ������˳����Q��W��ϵ����������ȷ����
A���������������� B�����Dz����Ͷ�Ԫ����
C����Ϊͬϵ�� D����Ϊͬ���칹��
��4��W�ϳɷ������£�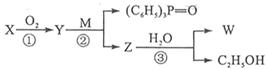
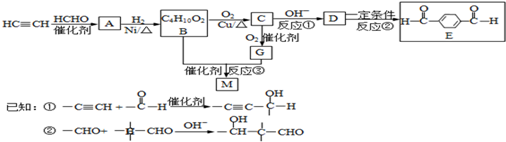
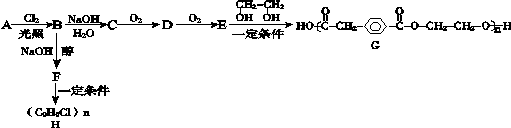
��֪����CHO + (C6H5)3P=CH��R����CH=CH��R + (C6H5)3P=O��R����ԭ�ӻ�ԭ���š�
���У� �ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
�ֱ����һ���л���ϳɹ�������������ͷ�Ӧ��������ȥ��
X��W��һ�������·�Ӧ����������N��N����Է�������Ϊ168��д��X�� W��һ�������·�Ӧ����N�Ļ�ѧ����ʽ�� ��
��5��д���ڢڲ���Ӧ�Ļ�ѧ����ʽ�� ��
��1��(3��) A C D ��2��(2��) 16 ��3��(2��) A
��4��HOCH2CH2OH+HOOC��CH=CH��CH=CH��COOH 
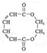 +2H2O(3��)
+2H2O(3��)
��5��OHC=CHO+2(C6H5)3P=CHCOOC2H5��2(C6H5)3P=O+H5C2OOCCH=CH��CH=CHCOOC2H5 (3��)
���������������1��WΪHOOC��CH=CH��CH=CH��COOH�����Ȼ�������ȡ����Ӧ��������Ӧ������̼̼˫�����ܷ����ӳɷ�Ӧ��������Ӧ��ѡA C D��
��2��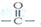 Ϊƽ��ṹ��������������ǻ�����CH=CH���棬������һ����CH=CH��COOHҲ���档����16��̼ԭ�ӡ�
Ϊƽ��ṹ��������������ǻ�����CH=CH���棬������һ����CH=CH��COOHҲ���档����16��̼ԭ�ӡ�
��3��A����ȷ��B������˫�������Dz����Ͷ�Ԫ���ᣬ����C���ṹ��ͬ������ͬϵ���ȷ��D��WΪ
C6H6O4;QΪC6H8O4������ʽ��ͬ������ͬ���칹�壬����
��4��X+W----N������+ H2O��WΪHOOC��CH=CH��CH=CH��COOH��NΪ168��XΪHOCH2CH2OH��
��һ�������·�Ӧ����������N��N����Է�������Ϊ168��
HOCH2CH2OH+HOOC��CH=CH��CH=CH��COOH 
 +2H2O
+2H2O
���㣺�������л��ƶ�Ϊ�����������л�����������ʵ�֪ʶ��

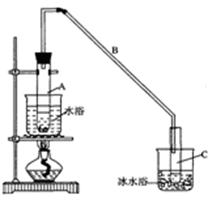
ij��ѧС���������������������װ�ã�����ͼ�����Ի������Ʊ�����ϩ
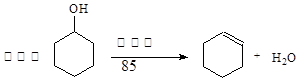
| | �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
��A�����Ƭ�������� �� ������B���˵�������е������� �� ��
���Թ�C���ڱ�ˮԡ�е�Ŀ���� �� ��
��2���Ʊ���Ʒ��
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ�� �� �㣨���ϻ��£�����Һ���� �� �����ţ�ϴ�ӻ���ϩ��
A.KMnO4��Һ B.ϡH2SO4 C.Na2CO3��Һ D.NaOH��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ�� �� ����f��g���ڽ��룬����ʱҪ������ʯ�ң�Ŀ���� �� ��
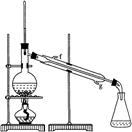
���ռ���Ʒʱ�����Ƶ��¶�Ӧ�� �� ���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���� �� ��
A.����ʱ��70�濪ʼ�ռ���Ʒ B.������ʵ����������
C.�Ʊ���Ʒʱ���������Ʒһ������
��3���������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������ķ����� �� ��
A.����KMnO4��Һ B.�ý����� C.�ⶨ�е� D.������Ȼ�̼��Һ
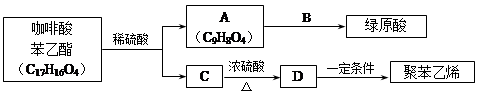
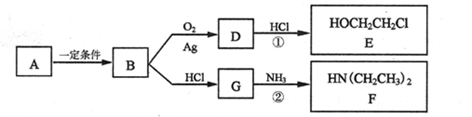
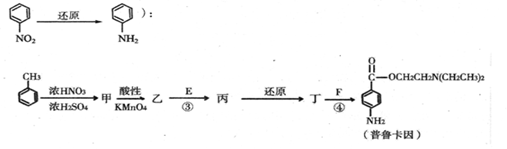
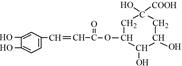 ����һ�ֿ�����ҩ�������ͼת����ϵ��
����һ�ֿ�����ҩ�������ͼת����ϵ��