��Ŀ����
NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��1����ҵ�Ͽ����Ȼ���Ϊԭ�ϣ�ͨ�����ķ����Ƶ�NaClO����ҵ����ȡNaClO�����ӷ�Ӧ����ʽΪ______ �������ҺPH______ 7������ڡ�С�ڡ����ڣ�����ԭ����______ �������ӷ���ʽ��ʾ��
��2��KAl��SO4��2��Һ�������غ��ʽΪ______
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl��SO4��2��Һ��Ϸ�Ӧ��ʵ�飮
�ٴ�������ƿ�м��뱥��KAl��SO4��2��Һ�����������İ�ɫ��״��������ʱ��Ӧ�����ӷ���ʽΪ______���ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ���ռ���һ����ɫ��ζ�����壮д���ڹ������»��Һ�з�Ӧ�Ļ�ѧ����ʽ��______��
��4��������Һ©���е�KAl��SO4��2��Һ������������泥�һ�ָ��Σ���NH4��2SO4?FeSO4����Һ���������䣮��Һ©����������ƿ�е��������������������Һ���۲쵽��ƿ���к��ɫ��������������û�й۲쵽����ɫ�����������ʱ��ƿ�з�����������ԭ��Ӧ�����ӷ���ʽΪ______��
��5��ȡ100mL 0.1mol/L Ba��OH��2��Һ����������μ���ͬŨ�ȵ�KHSO4��Һ��Ba2+ǡ����ȫ��������ʱ��Һ��PHֵΪ______ ����������Һ���ʱ������仯����Ϻ���Һ���¶�Ϊ100�棬100��ʱKw=1x10-12��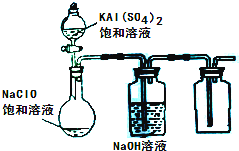
��1����ҵ�Ͽ����Ȼ���Ϊԭ�ϣ�ͨ�����ķ����Ƶ�NaClO����ҵ����ȡNaClO�����ӷ�Ӧ����ʽΪ______ �������ҺPH______ 7������ڡ�С�ڡ����ڣ�����ԭ����______ �������ӷ���ʽ��ʾ��
��2��KAl��SO4��2��Һ�������غ��ʽΪ______
��3��ijС��ͬѧ����ͼ��ʾװ��̽������NaClO��KAl��SO4��2��Һ��Ϸ�Ӧ��ʵ�飮
�ٴ�������ƿ�м��뱥��KAl��SO4��2��Һ�����������İ�ɫ��״��������ʱ��Ӧ�����ӷ���ʽΪ______���ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�����������ַ�Ӧ����ƿ���ռ���һ����ɫ��ζ�����壮д���ڹ������»��Һ�з�Ӧ�Ļ�ѧ����ʽ��______��
��4��������Һ©���е�KAl��SO4��2��Һ������������泥�һ�ָ��Σ���NH4��2SO4?FeSO4����Һ���������䣮��Һ©����������ƿ�е��������������������Һ���۲쵽��ƿ���к��ɫ��������������û�й۲쵽����ɫ�����������ʱ��ƿ�з�����������ԭ��Ӧ�����ӷ���ʽΪ______��
��5��ȡ100mL 0.1mol/L Ba��OH��2��Һ����������μ���ͬŨ�ȵ�KHSO4��Һ��Ba2+ǡ����ȫ��������ʱ��Һ��PHֵΪ______ ����������Һ���ʱ������仯����Ϻ���Һ���¶�Ϊ100�棬100��ʱKw=1x10-12��

��1����ⱥ��ʳ��ˮ�õ�����������������������Һ������������������Һ��Ӧ���ɴ������ƺ��Ȼ�����Һ������ȫ�����������Ʒ�Ӧ���ɴ���������Һ����������Ӧ�����ӷ���ʽΪ��Cl-+H2O
ClO-+H2��������������Һˮ���Լ��ԣ�ClO-+H2O?HClO+OH-���ʴ�Ϊ��Cl-+H2O
ClO-+H2�������ڣ�ClO-+H2O?HClO+OH-��
��2��Һ��������ˮ���������������������ӣ������غ���ˮ������������Ӻ������������غ㣬c��H+��=c��OH-��+3c��Al��OH��3�����ʴ�Ϊ��c��H+��=c��OH-��+3c��Al��OH��3����
��3������ƿ�м��뱥��KAl��SO4��2��Һ�������������ˮ���Լ��ԣ�������ˮ�������ԣ���Ϻ�ˮ����ٽ������������İ�ɫ��״�����ʹ����ᣬ��Ӧ�����ӷ���ʽΪ��
3Cl-+Al3++3H2O=3HClO+Al��OH��3�����ʴ�Ϊ��3Cl-+Al3++3H2O=3HClO+Al��OH��3����
�ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�������Ϊ��������ַ�Ӧ����ƿ���ռ���һ����ɫ��ζ������Ϊ����������������ԭ��Ӧ�����غ�д����ѧ����ʽ��ƽ�õ���4HClO
2H2O+2Cl2��+O2����
�ʴ�Ϊ��4HClO
2H2O+2Cl2��+O2����
��4��������Һ©���е�KAl��SO4��2��Һ������������泥�һ�ָ��Σ���NH4��2SO4?FeSO4����Һ���������䣮��Һ©����������ƿ�е��������������������Һ���۲쵽��ƿ���к��ɫ��������������û�й۲쵽����ɫ����������������Ӿ��л�ԭ�ԣ�����������ӽ�������������Һ�з���������ԭ��Ӧ��
��Ӧ�����ӷ���ʽΪ��3ClO-+6Fe2++3H2O=2Fe��OH��3��+4Fe3++3Cl-��
�ʴ�Ϊ��3ClO-+6Fe2++3H2O=2Fe��OH��3��+4Fe3++3Cl-��
��5��ȡ100mL 0.1mol/L Ba��OH��2��Һ����������μ���ͬŨ�ȵ�KHSO4��Һ��Ba2+ǡ����ȫ������Ba��OH��2��Һ��KHSO4��Һ��Ba2+ǡ����ȫ��������ҪͬŨ����Һ���Ϊ100ml����Ӧ���ʵ���֮��Ϊ1��1��ʣ���������������ʵ���Ϊ0.01mol��Ũ��c��OH-��=
=0.05mol/L��100��ʱKw=1x10-12��c��H+��=
=2��10-11mol/L��PH=-lg2��10-11mol/L=10.7���ʴ�Ϊ��10.7��
| ||
| ||
��2��Һ��������ˮ���������������������ӣ������غ���ˮ������������Ӻ������������غ㣬c��H+��=c��OH-��+3c��Al��OH��3�����ʴ�Ϊ��c��H+��=c��OH-��+3c��Al��OH��3����
��3������ƿ�м��뱥��KAl��SO4��2��Һ�������������ˮ���Լ��ԣ�������ˮ�������ԣ���Ϻ�ˮ����ٽ������������İ�ɫ��״�����ʹ����ᣬ��Ӧ�����ӷ���ʽΪ��
3Cl-+Al3++3H2O=3HClO+Al��OH��3�����ʴ�Ϊ��3Cl-+Al3++3H2O=3HClO+Al��OH��3����
�ڽ���ƿ�еĻ��Һ�����������£�������ƿ���л���ɫ�������Ϊ��������ַ�Ӧ����ƿ���ռ���һ����ɫ��ζ������Ϊ����������������ԭ��Ӧ�����غ�д����ѧ����ʽ��ƽ�õ���4HClO
| ||
�ʴ�Ϊ��4HClO
| ||
��4��������Һ©���е�KAl��SO4��2��Һ������������泥�һ�ָ��Σ���NH4��2SO4?FeSO4����Һ���������䣮��Һ©����������ƿ�е��������������������Һ���۲쵽��ƿ���к��ɫ��������������û�й۲쵽����ɫ����������������Ӿ��л�ԭ�ԣ�����������ӽ�������������Һ�з���������ԭ��Ӧ��
��Ӧ�����ӷ���ʽΪ��3ClO-+6Fe2++3H2O=2Fe��OH��3��+4Fe3++3Cl-��
�ʴ�Ϊ��3ClO-+6Fe2++3H2O=2Fe��OH��3��+4Fe3++3Cl-��
��5��ȡ100mL 0.1mol/L Ba��OH��2��Һ����������μ���ͬŨ�ȵ�KHSO4��Һ��Ba2+ǡ����ȫ������Ba��OH��2��Һ��KHSO4��Һ��Ba2+ǡ����ȫ��������ҪͬŨ����Һ���Ϊ100ml����Ӧ���ʵ���֮��Ϊ1��1��ʣ���������������ʵ���Ϊ0.01mol��Ũ��c��OH-��=
| 0.01mol |
| 0.2L |
| 10-12 |
| 0.05 |

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ
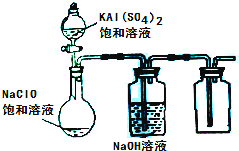 ��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
��2013?����ģ�⣩NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��

