��Ŀ����
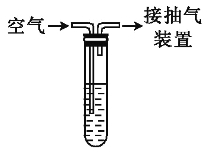
����Ŀ�����������壨NiSO47H2O�������ڵ�ƹ�ҵ�����ú����ϴ���Ϊԭ�����Ʊ�����֪ij�������ĺ����ϴ�����Ҫ����Ni��������Al��Fe�ĵ��ʼ������������ʣ�����������ijС��ͨ���������ϣ����������ͼ��ʾ���Ʊ����̣�
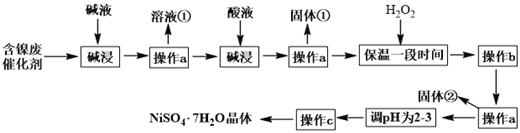
��֪�� Ksp[Fe(OH)3]=4.0��10-38 ��Ksp[Ni(OH)2]=1.2��10-15
��1��������������з�����Ӧ�����ӷ���ʽ��_______________________________��
��2������a���õ��IJ����������ձ���________��________������c������Ϊ____________��____________�����ˡ�ϴ�ӡ�
��3���������______________����H2O2��Ŀ���ǣ������ӷ���ʽ��ʾ��_____________��
��4����pHΪ2-3ʱ���ӵ�����________��
��5������bΪ������Һ��pH������������b����Һ��c��Ni2����=2mol��L-1����������ǡ����ȫ������Һ��c��Fe3����=1.0��10-5mol��L-1ʱ����Һ���Ƿ���Ni(OH)2�������ɣ�________����ǡ�����
��6��NiSO47H2O�������Ʊ������أ�NiMH����������Ŀǰ�Ѿ���Ϊ��϶���������һ����Ҫ������ͣ�NiMH�е�M��ʾ���������Ͻ𣮸õ���ڷŵ�������ܷ�Ӧ�Ļ�ѧ����ʽ��NiOOH+MH=Ni(OH)2+M����NiMH��س������У������ĵ缫��ӦʽΪ_______��
���𰸡� 2Al+2OH-+2H2O�T2AlO2-+3H2�� ©�� ������ ����Ũ�� ��ȴ�ᾧ �������� 2Fe2++H2O2+2H+=2Fe3++2H2O ���ᣨH2SO4�� �� Ni(OH)2 + OH- - e- = NiOOH + H2O
����������1��������Ʒ��ֻ�н�������ǿ����Һ������Ӧ����ƫ�����κ����������ӷ���ʽΪ��2Al+2OH-+2H2O�T2AlO2-+3H2������ȷ�𰸣�2Al+2OH-+2H2O�T2AlO2-+3H2����
��2������aΪ�������õ������������ձ���©�� ��������������Һ�еõ����������壨NiSO47H2O��������Ҫ�Ƴɸ���ʱ������Һ��Ȼ��������Ũ������ȴ�ᾧ�����ˡ�ϴ������ȷ����©�� ��������������Ũ������ȴ�ᾧ��
��3�������������ᣬֻ�����ʲ�������ͼ���Թ�����Dz������ʣ���Һ�д������������ӣ�����H2O2��Ŀ�ľ��������������ӱ�Ϊ�����ӣ����ӷ���ʽΪ��2Fe2++H2O2+2H+=2Fe3++2H2O����ȷ�𰸣��������ʣ�2Fe2++H2O2+2H+=2Fe3++2H2O��
��4�����Ҫ�õ�NiSO47H2O��Ϊ�˲������������ӣ���ü����������Һ��pH����ȷ�𰸣����ᣨH2SO4����
��5������֪ Ksp[Fe(OH)3]=4.0��10-38 ��Ksp[Ni(OH)2]=1.2��10-15������Ksp[Fe(OH)3]=c(Fe3+)��c3(OH-)=1.0��10-5��c3(OH-)=4.0��10-38, c3(OH-)=4.0��10-33 mol��L-1, c(OH-)=1.6��10-11 mol��L-1, Ni(OH)2��Ũ����QC= c(Ni2+)��c2(OH-)=2����1.6��10-11��2=5.12��10-22< Ksp[Ni(OH)2]=1.2��10-15����Һ��û��Ni(OH)2�������ɣ���ȷ�𰸣���
��6���ŵ�Ϊԭ��أ����Ϊ���أ�������������������Ӧ��Ni(OH)2��NiԪ����+2������ΪNiOOH�е�+3�ۣ�����������Ӧ�������ĵ缫��ӦʽΪNi(OH)2 + OH- - e- = NiOOH + H2O����ȷ�𰸣�Ni(OH)2 + OH- - e- = NiOOH + H2O��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�