��Ŀ����
����Ŀ������ѧѡ��3:���ʽṹ�����ʡ�A��B��C��D��E����Ԫ�ص�ԭ�����������������зǽ���Ԫ��A�Ļ�̬ԭ���гɶԵ�������δ��ʱ��������������CԪ���ڵؿ��к�����ߣ�D�ĵ����Ƕ��������۵���͵Ľ�����E�ĺϽ����ҹ�ʹ������ĺϽ�
��1��EԪ�صĻ�̬ԭ�ӵ����Ų�ʽΪ__________________��
��2��A��ij���⻯��A2H2�����к���___���Ҽ���____���м���
��3��A �ĺ����������AO3n-�Ŀռ乹����___________��
��4��B������⻯��ķе��A ������⻯��ķе�ߵö࣬��ԭ����_____��
��5��E������������Ӧ��ˮ�����ܽ��ڰ�ˮ�����ɵĸ��ӻ�����Ļ�ѧʽ��______��
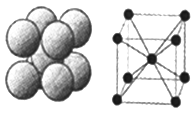
��6����ͼ��D���ʵľ���ѻ���ʽ�����ֶѻ���ʽ�ľ�����ԭ�ӵ���λ��Ϊ____������ԭ�ӵİ뾶Ϊrpm ���˾�����ܶȦ�=______g/cm3���ú�r�Ĵ���ʽ��ʾ������٤��������NA��ʾ����
���𰸡� 1s22s22p63s23p63d104s1 3 2 �������� �����Ӽ�����γ������������Ӽ䲻�ܣ������ķе�ȼ���� [Cu(NH3)4](OH)2 8 ![]()
�����������������A��B��C��D��E����Ԫ�ص�ԭ��������������CԪ���ڵؿ��к�����ߣ�C����Ԫ�ء����зǽ���Ԫ��A�Ļ�̬ԭ���гɶԵ�������δ��ʱ��������������ԭ������С����Ԫ�أ���A��̼Ԫ�أ�����B�ǵ�Ԫ�ء�D�ĵ����Ƕ��������۵���͵Ľ�������D���ơ�E�ĺϽ����ҹ�ʹ������ĺϽ���E��ͭ���ݴ˷������
������A��B��C��D��E����Ԫ�ص�ԭ��������������CԪ���ڵؿ��к�����ߣ�C����Ԫ�ء����зǽ���Ԫ��A�Ļ�̬ԭ���гɶԵ�������δ��ʱ��������������ԭ������С����Ԫ�أ���A��̼Ԫ�أ�����B�ǵ�Ԫ�ء�D�ĵ����Ƕ��������۵���͵Ľ�������D���ơ�E�ĺϽ����ҹ�ʹ������ĺϽ���E��ͭ��
��1��ͭ��ԭ��������29��ͭԪ�صĻ�̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
��2��A��ij���⻯��A2H2��������Ȳ����Ȳ�ĽṹʽΪH��C��C��H��������к���3��������2��������
��3��A �ĺ����������CO32-��̼ԭ�ӵļ۲���Ӷ�����![]() ������̼����Ŀռ乹����ƽ���������Ρ�
������̼����Ŀռ乹����ƽ���������Ρ�
��4�������Ӽ�����γ������������Ӽ䲻�ܣ������ķе�ȼ������
��5��ͭ������������Ӧ��ˮ����������ͭ�ܽ��ڰ�ˮ�����ɵĸ��ӻ�����Ļ�ѧʽ��[Cu(NH3)4](OH)2��
��6�����ݼصľ����ṹ��֪���ֶѻ���ʽ�ľ�����ԭ�ӵ���λ��Ϊ8������ԭ�ӵİ뾶Ϊrpm�������ĵĶԽ�����4rpm�����Ծ����ı߳���![]() ���þ����������
���þ����������![]() �������к���2����ԭ�ӣ���˾�����ܶ�����
�������к���2����ԭ�ӣ���˾�����ܶ�����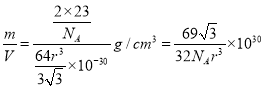 g/cm3��
g/cm3��

����Ŀ����X��Y��Z��T���ֶ�����Ԫ�ء�X��Y��Z��Ԫ�������ڱ��е�λ����ͼ��ʾ����Ԫ�ص�ԭ������֮����41��X��T�ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���������ֻ����
X | |
Y | Z |
(1)��Ԫ�صķ�����X______��Y________��Z________��T___________��
(2)Yԭ�ӵĽṹʾ��ͼΪ______________________________________________��
(3)�õ���ʽ��ʾY��T��ɵĻ�������γɹ��̣�_____________________��
(4)X��Y���⻯����ȶ���_________���û�ѧʽ��ʾ����
����Ŀ����10L�����ܱ������г���X(g)��Y(g)��������ӦX(g)��Y(g)![]() M(g)��N(g)
M(g)��N(g)
ʵ���� | �¶�/�� | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | |
n(X) | n(Y) | n(M) | ||
�� | 700 | 0.40 | 0.10 | 0.090 |
�� | 800 | 0.10 | 0.40 | 0.080 |
�� | 800 | 0.20 | 0.20 | a |
�� | 800 | 0.10 | 0.10 | b |
����˵����ȷ���ǡ�(����)
A. ʵ����У���5minʱ���n(M)��0.050mol����0��5minʱ���ڣ���N��ʾ��ƽ����Ӧ���ʦ�(N)��1.0��10��2mol/(L��min)
B. ʵ����У��÷�Ӧ��ƽ�ⳣ��K��2.0
C. ʵ����У��ﵽƽ��ʱ��X��ת����Ϊ50%
D. ʵ����У��ﵽƽ��ʱ��b<0.05