��Ŀ����
20�����У�CO${\;}_{3}^{2-}$��NH${\;}_{4}^{+}$��Al3+��ClO-��Na+��SO${\;}_{4}^{2-}$��AlO${\;}_{2}^{-}$��Ba2+��HCO${\;}_{2}^{-}$��Cl-�����ӣ��ش��������⣺��1����ˮ��Һ�У�����ˮ��ʼ��Ե���CO32-��ClO-��AlO2-��HCO3-����
��2����ˮ��Һ�У�����ˮ������Ե���NH4+��Al3+��
��3���Ȳ��������Խ�ǿ����Һ��������ڣ��ֲ����ڼ��Խ�ǿ����Һ��������ڵ�������HCO3-��
��4������������ӷ���ʽ��3CO${\;}_{3}^{2-}$+2Al3++3H2O�T2Al��OH��3��+3CO2 ����
���� ��1�����������ˮ���Լ��ԣ�
��2������������ˮ������ʽ��
��3����Ԫ�������ʽ�������������ӡ����������Ӷ���Ӧ�������ܹ��棻
��4��̼��������������ӷ���˫ˮ�����ɶ�����̼������������
��� �⣺CO32-��ClO-��AlO2-��HCO3-��Ϊ��������ӣ�ˮ���Լ��ԣ�
NH4+��Al3+��Ϊ���������ӣ�ˮ�������ԣ�
Na+��SO42-��Ba2+��Cl-�����ӣ����ܷ���ˮ�⣻
��1��CO32-��ClO-��AlO2-��HCO3-��Ϊ��������ӣ�ˮ���Լ��ԣ�
�ʴ�Ϊ��CO32-��ClO-��AlO2-��HCO3-��
��2��NH4+��Al3+��Ϊ���������ӣ�ˮ�������ԣ�
�ʴ�Ϊ��NH4+��Al3+��
��3��HCO3-Ϊ��Ԫ�������ʽ�������������ӡ����������Ӷ���Ӧ�������ܹ��棻
�ʴ�Ϊ��HCO3-��
��4��̼��������������ӷ���˫ˮ�����ɶ�����̼������������ˮ������ӷ���ʽ��3CO32-+2Al3++3H2O�T2Al��OH��3��+3CO2 ����
�ʴ�Ϊ��3CO32-+2Al3++3H2O�T2Al��OH��3��+3CO2 ����
���� ���⿼��������ˮ�⣬��ȷ����ˮ������ǽ���ؼ���ע��˫ˮ�⳹��Ӧ��������ϣ��õȺţ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ
11�����нṹͼ�У������ǰ������Ԫ�ص�ԭ��ʵ��ԭ��ʵ��ԭ�ӳ�ȥ�������Ӻ�ʣ��IJ��֣���С�ڵ����δ�����γɹ��ۼ����������ӣ����ߴ����ۼ���ʾ����

���ݸ�ͼ��ʾ�Ľṹ�ص㣬�����й�������ȷ���ǣ�������


���ݸ�ͼ��ʾ�Ľṹ�ص㣬�����й�������ȷ���ǣ�������
| A�� | ͨ��״������ˮ���ܽ�ȱ����ڶ� | |
| B�� | �ס��ҡ���Ϊ�Ǽ��Է��ӣ���Ϊ���Է��� | |
| C�� | ��ˮ��Һ�д���4����� | |
| D�� | ���е縺��С��Ԫ������е縺�Դ��Ԫ���γɵĻ�����һ�������ڼ��Լ� |
12���Է�Ӧ��2X��g��+Y��g��?2Z��g������Сѹǿʱ���Է�Ӧ������Ӱ���ǣ�������
| A�� | �淴Ӧ������������Ӧ���ʼ�С | B�� | �淴Ӧ���ʼ�С������Ӧ�������� | ||
| C�� | �����淴Ӧ���ʶ���С | D�� | �����淴Ӧ���ʶ����� |
 ʵ���ҿ���������ͼ��ʾװ���Ʊ�������
ʵ���ҿ���������ͼ��ʾװ���Ʊ�������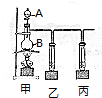 ijѧϰС��Ϊ̽������Ԫ�ػ���������ʣ�����������ʵ��װ�ã�
ijѧϰС��Ϊ̽������Ԫ�ػ���������ʣ�����������ʵ��װ�ã�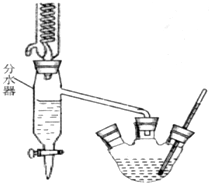 ��֪�����������ķе�Ϊ213�棨�ڴ��¶�����ˮ���Ҵ��ͻ�������7.0%��17.0%��76.0%�ı�����Ϊ�����ݳ�������ش�����ʵ�����Ʊ��������������й����⣺
��֪�����������ķе�Ϊ213�棨�ڴ��¶�����ˮ���Ҵ��ͻ�������7.0%��17.0%��76.0%�ı�����Ϊ�����ݳ�������ش�����ʵ�����Ʊ��������������й����⣺