��Ŀ����
8�������仯���������������������Ź㷺��Ӧ�ã���1����ͼ3���о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ����ͼ��A��B��C��D�ĸ���λ�У������������IJ�λ��B������ĸ����
��2����֪t��ʱ����ӦFeO��s��+CO��g��?Fe��s��+CO2��g����ƽ�ⳣ��K=0.25����÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��}{c��CO��}$��t��ʱ����Ӧ�ﵽƽ��ʱn��CO����n��CO2��=4��t��ʱ������1L�ܱ������м���0.02molFeO��s����xmolCO��ƽ��ʱFeO��ת����Ϊ50%����x=0.05��
��3�����������һ�ָ�Ч�Ķ�ܵ�ˮ����������ҵ�ϳ�����NaClO�������������йط�Ӧԭ��Ϊ��
3NaClO+2Fe��NO3��3+10NaOH�T2Na2FeO4��+3NaCl+6NaNO3+5H2O
Na2FeO4+2KOH�TK2FeO4+2NaOH
ʵ��֤������Ӧ���¶ȡ�ԭ�ϵ�Ũ�ȼ���ȶԸ�����صIJ��ʶ���Ӱ�죮ͼ1Ϊ��ͬ�¶��£�Fe��NO3��3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죮ͼ2Ϊһ���¶��£�NaClO��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죮
�ٹ�ҵ�����У���Ӧ���е������¶�Ϊ26�棻��ʱFe��NO3��3��NaClO������Һ�����������Ũ��֮����6��5��
�ڸ��������ˮ������ʱ��������Ҫ��ɱ��������������������2�����ɣ���

���� ��1�����Ի������������£�������������ʴ�����Ӵ�������ˮʱ��ʴ�����أ�
��2��ƽ�ⳣ����ָ������ƽ��Ũ�Ȼ�ѧ�������ݳ˻��뷴Ӧ��ƽ��Ũ�Ȼ�ѧ�������ݳ˻��ıȣ�
����ƽ�ⳣ������ʽ��֪����Ӧ�ﵽƽ��ʱn��CO����n��CO2������ƽ�ⳣ���ĵ�����
��x��ʾ��ƽ��ʱCO��������̼���ʵ������ٸ���ƽ�ⳣ���з��̼�����
��3������ͼ1��֪��Fe��NO3��3Ũ��һ�����¶���26��ʱ��K2FeO4����������ߣ�Fe��NO3��3Ũ����330g/Lʱ��K2FeO4����������ߣ���ͼ2��֪��NaClO��275g/Lʱ��K2FeO4����������ߣ��ݴ˼���Fe��NO3��3��NaClO������Һ�������Ũ��֮�ȣ�
�ڸ�����ؾ���ǿ�����ԣ�����ɱ����������ԭ�õ�������ˮ���γ������������壬��������ˮ�������
��� �⣺��1����ˮ��Һ�����ԣ����������������ʴ�������Ӵ�������ˮʱ��ʴ�����أ�����B����ʴ�����أ��ʴ�Ϊ��B��
��2����ӦFeO��s��+CO��g��?Fe��s��+CO2��g����ƽ�ⳣ��K=$\frac{c��C{O}_{2}��}{c��CO��}$��t��ʱ����Ӧ�ﵽƽ��ʱn��CO����n��CO2��=$\frac{1}{K}$=$\frac{1}{0.25}$=4��t��ʱ��
����1L�ܱ������м���0.02molFeO��s����xmolCO��ƽ��ʱFeO��ת����Ϊ50%��ת����FeOΪ0.01mol����
FeO��s��+CO��g��?Fe��s��+CO2��g��
��ʼ����mol����0.02 x 0
�仯����mol����0.01 0.01 0.01
ƽ������mol����0.01 x-0.01 0.01
��$\frac{0.01}{x-0.01}$=0.25�����x=0.05��
�ʴ�Ϊ��$\frac{c��C{O}_{2}��}{c��CO��}$��4��0.05��
��3������ͼ1��֪��Fe��NO3��3Ũ��һ�����¶���26��ʱ��K2FeO4����������ߣ��ʹ�ҵ����������¶�Ϊ26�森
��ͼ1��֪��Fe��NO3��3Ũ����330g/Lʱ��K2FeO4����������ߣ���ͼ2��֪��NaClO��275g/Lʱ��K2FeO4����������ߣ�����Fe��NO3��3��NaClO������Һ�������Ũ��֮��Ϊ330g/L��275g/L=6��5��
�ʴ�Ϊ��26��6��5��
�ڸ�����ؾ���ǿ�����ԣ�����ɱ����������ԭ�õ�������ˮ���γ������������壬��������ˮ�������
�ʴ�Ϊ��ɱ�����������������
���� ���⿼�黯ѧƽ����㡢������ʴ����������ѡ��ȣ��Ѷ��еȣ���Ҫѧ��������ʵ�Ļ���֪ʶ���������֪ʶ���Ķ���ȡ��Ϣ��������������������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 115 | B�� | 49 | C�� | 66 | D�� | 164 |
| A�� |  ͭ��ұ�� | B�� | 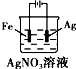 ���϶��� | C�� |  ��ֹFe����ʴ | D�� |  ����ͭпԭ��� |
| A�� | ��ҵ��ȡ����ʱβ��������������Һ���� | |
| B�� | ����й¶ʱ��Ӧ��պ�з���ˮ��ʪë����ס�ڱǵ���ȫ���� | |
| C�� | ��������������ʱ���Ͻ�Ư����Ũ������ʹ�ã����������ж����� | |
| D�� | ���з�����Ȼ��й¶ʱ��Ӧ����ʹ�����ڵ绰���� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | �������ὦ�����У�Ӧ������ˮ��ϴ����ϴ��գ�۾� | |
| B�� | ����������Һʱ����������Ͳ�м���һ�������ˮ�����ڽ�����������������Ũ���� | |
| C�� | �ƾ����Ż�ʱ����ɳ������ | |
| D�� | ������Ũ����Һմ��Ƥ���ϣ�Ҫ�����ô���ˮ��ϴ��Ȼ��Ϳ������ |
 ��֪���屽���۵�Ϊ?30.8�棬�е�156�森ʵ�����ñ���Һ����ȡ�屽��װ����ͼ��
��֪���屽���۵�Ϊ?30.8�棬�е�156�森ʵ�����ñ���Һ����ȡ�屽��װ����ͼ��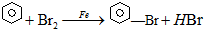 ����
����