��Ŀ����
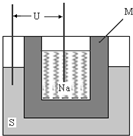 ��2011?ɽ�������С������г��漰�ơ����仯���
��2011?ɽ�������С������г��漰�ơ����仯�����1��ʵ��������ˮ�Ҵ��������������Ľ����ƣ���ѧ����ʽΪ
2CH3CH2OH+2Na=2CH3CH2ONa+H2��
2CH3CH2OH+2Na=2CH3CH2ONa+H2��
��Ҫ��ϴ�������Թܱ��ϵ����õ��Լ���CS2[���ȣ�NaOH��Һ]
CS2[���ȣ�NaOH��Һ]
����2����ͼΪ������ܵ�صĽṹʾ��ͼ���õ�صĹ����¶�Ϊ200�����ң���ط�ӦΪ
2Na+xS=Na2Sx�������ĵ缫��ӦʽΪ
xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx��
xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx��
��M����Na2O��Al2O3�Ƶã����������������ӵ��磨��������ʣ�����������
���ӵ��磨��������ʣ�����������
����Ǧ������ȣ���������ͬ�����ĸ�����������ʱ�������ص����۷ŵ�����Ǧ����4.5
4.5
������3��Na2S��Һ������Ũ���ɴﵽС��˳��Ϊ
c��Na+����c��S2-����c��OH-����c��HS-����c��H+��
c��Na+����c��S2-����c��OH-����c��HS-����c��H+��
�������Һ�м�����������CuSO4����ҺpH��С
��С
�����������С�����䡱���� Na2S��Һ���ڷ�����������������Ϊ2S2-+O2+2H2O=2S��+4OH-
2S2-+O2+2H2O=2S��+4OH-
�������ӷ���ʽ��ʾ������������1�������Ҵ��ܺ��Ʒ�Ӧ�������Լ����ڶ���̼�е��ܽ���֪ʶ���ش�
��2��ԭ������������õ��ӵĻ�ԭ��Ӧ�����ԭ��صĹ�������������ʵ�����������M�����ã�����Ǧ���صĹ���ԭ����������ܵ�صĹ���ԭ�����ش�
��3�������εĵ�������ӵ�ˮ��֪ʶ���Ƚ�����Ũ�ȴ�С�����ݼ�������ͭ���������ķ�Ӧ������ж�pH�ı仯�����������ӵ�ǿ��ԭ�����ش�
��2��ԭ������������õ��ӵĻ�ԭ��Ӧ�����ԭ��صĹ�������������ʵ�����������M�����ã�����Ǧ���صĹ���ԭ����������ܵ�صĹ���ԭ�����ش�
��3�������εĵ�������ӵ�ˮ��֪ʶ���Ƚ�����Ũ�ȴ�С�����ݼ�������ͭ���������ķ�Ӧ������ж�pH�ı仯�����������ӵ�ǿ��ԭ�����ش�
����⣺��1���Ҵ��ܺ��Ʒ�Ӧ������ʽΪ��2CH3CH2OH+2Na=2CH3CH2ONa+H2�����������ڶ���̼�����ھƾ���������ˮ�����ܺ��ȵ�����������Һ��Ӧ���ʴ�Ϊ��2CH3CH2OH+2Na=2CH3CH2ONa+H2����CS2[���ȣ�NaOH��Һ]��
��2��ԭ������������õ��ӵĻ�ԭ��Ӧ���ڷ�Ӧ2Na+xS=Na2Sx�У����ʵõ��ӣ���������ӦΪ��xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx����M��Ϊ����ʵ�ͬʱ�ֽ��ƺ����������Ǧ������ȣ���������ͬ�����ĸ�����������Ǧ����ʱ��Ǧ��ΪǦ����ʱת�Ƶ��ӵ����ʵ������Ƴ�Ϊ������ʱת�Ƶĵ��ӵ����ʵ�����4.5�����������ص����۷ŵ�����Ǧ����4.5�����ʴ�Ϊ��xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx�������ӵ��磨��������ʣ�����������4.5��
��3�����Ƶ�����������������Ӻ������ӣ�������������Ũ���������ӵ�2������c��Na+����c��S2-������������������ˮ�⣬��һ��ˮ��ǿ�ڵڶ���������ˮ�����������������c��S2-����c��OH-����c��HS-������Һ�Լ��ԣ���������Ũ����С��������ͭ����Һ�ɼ���������Һ��Ϊ������������Һ����pH ��С���ʴ�Ϊ��c��Na+����c��S2-����c��OH-����c��HS-����c��H+������С��
��4���������к�ǿ��ԭ�ԣ������ױ�������������Ӧ����ʽΪ��2S2-+O2+2H2O=2S��+4OH-���ʴ�Ϊ��2S2-+O2+2H2O=2S��+4OH-��
��2��ԭ������������õ��ӵĻ�ԭ��Ӧ���ڷ�Ӧ2Na+xS=Na2Sx�У����ʵõ��ӣ���������ӦΪ��xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx����M��Ϊ����ʵ�ͬʱ�ֽ��ƺ����������Ǧ������ȣ���������ͬ�����ĸ�����������Ǧ����ʱ��Ǧ��ΪǦ����ʱת�Ƶ��ӵ����ʵ������Ƴ�Ϊ������ʱת�Ƶĵ��ӵ����ʵ�����4.5�����������ص����۷ŵ�����Ǧ����4.5�����ʴ�Ϊ��xS+2e-=Sx2-����2Na++xS+2e-=Na2Sx�������ӵ��磨��������ʣ�����������4.5��
��3�����Ƶ�����������������Ӻ������ӣ�������������Ũ���������ӵ�2������c��Na+����c��S2-������������������ˮ�⣬��һ��ˮ��ǿ�ڵڶ���������ˮ�����������������c��S2-����c��OH-����c��HS-������Һ�Լ��ԣ���������Ũ����С��������ͭ����Һ�ɼ���������Һ��Ϊ������������Һ����pH ��С���ʴ�Ϊ��c��Na+����c��S2-����c��OH-����c��HS-����c��H+������С��
��4���������к�ǿ��ԭ�ԣ������ױ�������������Ӧ����ʽΪ��2S2-+O2+2H2O=2S��+4OH-���ʴ�Ϊ��2S2-+O2+2H2O=2S��+4OH-��
������������һ�������Ե���Ŀ��ͬʱ���������л���֪ʶ���绯ѧ֪ʶ����������Һ��ˮ��͵���֪ʶ���ѶȺܴ�

��ϰ��ϵ�д�
�����Ŀ
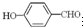 ��
�е��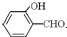 �ߣ�ԭ����
�ߣ�ԭ����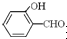 �γɷ������������
�γɷ������������ �γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������
�γɷ��Ӽ���������Ӽ����ʹ���Ӽ�����������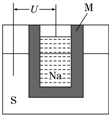 ��1����2011?���ո߿�����ѡ��Ag2O2����п���Ե�ص������������ʣ���������ҺΪ KOH ��Һ����طŵ�ʱ������Ag2O2ת��ΪAg��������Znת��ΪK2Zn��OH��4��д���õ�ط�Ӧ����ʽ��
��1����2011?���ո߿�����ѡ��Ag2O2����п���Ե�ص������������ʣ���������ҺΪ KOH ��Һ����طŵ�ʱ������Ag2O2ת��ΪAg��������Znת��ΪK2Zn��OH��4��д���õ�ط�Ӧ����ʽ��
