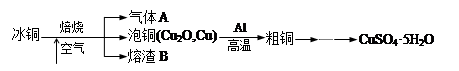��Ŀ����
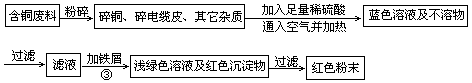
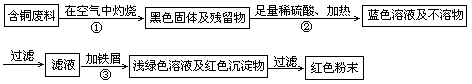
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ�������ͼ��
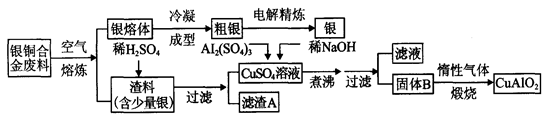
��1����⾫����ʱ��������ӦʽΪ_______________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���ú���ɫ������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2����������B�����Ϊ_____________�������ɹ���B�Ĺ����У��������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ_____________________��
��3�����չ��������ɵ�����������NH3�ڴ��������·�Ӧ�Ļ�ѧ����ʽΪ_____________________�������Ӧ�л��а��̲������ð���Ϊ______________��
��4������ͭ�Ͻ���ͭ����������Ϊ64����������3��0kg�����е�ͭ����ȫת��Ϊ__________molCuAlO2��������Ҫ1��0 mol��L��1��Al2(SO4)3��Һ___________L��

��1����⾫����ʱ��������ӦʽΪ_______________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���ú���ɫ������ˮ��Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
��2����������B�����Ϊ_____________�������ɹ���B�Ĺ����У��������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ_____________________��
��3�����չ��������ɵ�����������NH3�ڴ��������·�Ӧ�Ļ�ѧ����ʽΪ_____________________�������Ӧ�л��а��̲������ð���Ϊ______________��
��4������ͭ�Ͻ���ͭ����������Ϊ64����������3��0kg�����е�ͭ����ȫת��Ϊ__________molCuAlO2��������Ҫ1��0 mol��L��1��Al2(SO4)3��Һ___________L��
( ��10�֣�(1)Ag+ + e����Ag ��1�֣� 3NO2 +H2O��2HNO3 + NO�� (1�֣�
��2��CuO����Cu(OH)2�� ��Al(OH)3��1�֣� OH�D +Al(OH)3��AlO2-+2H2O��1�֣�
��3��4NH3+5O2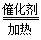 4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣�
4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣�
��2��CuO����Cu(OH)2�� ��Al(OH)3��1�֣� OH�D +Al(OH)3��AlO2-+2H2O��1�֣�
��3��4NH3+5O2
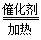 4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣�
4NO+6H2O��1�֣� NH4NO3��1�֣� (4)30 ��1�֣���15 ��1�֣������������1�����վ���ͭ��ԭ������ȷ��������������Ag��e���� Ag+��������������Ag++e���� Ag������A�еĽ�����ϡ���ᷴӦ������ɫ��NO��NO������е�������Ӧ���ɺ���ɫ��NO2��NO2��ˮ��Ӧ���������NO����Ӧ�Ļ�ѧ����ʽΪ3NO2 +H2O��2HNO3 + NO����
��2�������Ϣ������ͼ������֪������ͭ��������������ϡ�������Ʒ�Ӧ����������ͭ���������������ʱ������ͭ�ֽ�ΪCuO�������������ֽ⣬����BӦ��ΪCuO��Al(OH)3���������������������������������NaOH��������������������Al(OH)3�ͻ��ܽ⣬��Ӧ�����ӷ���ʽΪAl(OH)3+OH����AlO��+2H2O��
��3���ڴ����������£�������������������NO��ˮ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
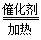 4NO+6H2O�����ɵ�NO��������ˮ���������������ᣬ�����백����Ӧ��������臨�ð���̡�
4NO+6H2O�����ɵ�NO��������ˮ���������������ᣬ�����백����Ӧ��������臨�ð���̡���4����ͭ�Ͻ��е�ͭ�����ʵ���n(Cu)��
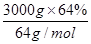 ��30mol������Ԫ���غ�ɵ����ɵ�CuAlO2Ҳ��30.0mol�����ݻ�ѧʽCuAlO2�е�Cu��Al������ϵ��Alԭ�Ӹ����غ�ɵ�,n[Al2(SO4)3]�� 30.0mol��2��15.0mol��������Ҫ��������Һ�������15.0mol��1.0mol/L��15.0L��
��30mol������Ԫ���غ�ɵ����ɵ�CuAlO2Ҳ��30.0mol�����ݻ�ѧʽCuAlO2�е�Cu��Al������ϵ��Alԭ�Ӹ����غ�ɵ�,n[Al2(SO4)3]�� 30.0mol��2��15.0mol��������Ҫ��������Һ�������15.0mol��1.0mol/L��15.0L��
��ϰ��ϵ�д�
�����Ŀ




 2CuSO4+2H2O ) ���������������һ������
2CuSO4+2H2O ) ���������������һ������