��Ŀ����
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��

����ɸ�ʵ���е����⡣
(1)д���÷�Ӧ�ķ�Ӧ����ʽ��________________________________________��
��ָ����������ԭ��Ӧ�Ļ�ԭ����___________����������___________��
(2)ʵ��ǰ���������װ�ý��������Լ�飬����������____________________��
(3)Բ����ƿ��ʢװ��ˮ����װ�����Ⱥ����Ҫ������______________________________����ƿ�ײ������˼�Ƭ���Ƭ�����Ƭ��������____________________��
(4)�ƾ��ƺ;ƾ���Ƶ�ȼ��˳����__________��Ϊʲô��__________________________��
(5)�������ʢװ��������__________��������____________________________________��
(6)�Թ����ռ�������___________�����Ҫ��A�������ܴ���ȼ�����壬�����Ը��������__________��������________________________________________����һ������Ŀ����_______________________��
(1)3Fe+4H2O(g)![]() Fe3O4+4H2 Fe H2O
Fe3O4+4H2 Fe H2O
(2)�ڲ����ܿڴ���һ���齺�ܣ��齺��ͷ����һ�β����ܣ������ܿ�û��ˮ�У��þƾ��ƻ�������ƿ�ײ�������ܹ��۲쵽û��ˮ�еIJ����ܿ��������ݳ���ֹͣ���Ⱥ���������ˮ���������ҽϳ�ʱ��ˮ�������䣬���������װ������������
(3)ΪӲ�ʲ�������Fe��ˮ�����ķ�Ӧʵ���ṩ�������ϵ�ˮ���� ��ֹ�����¹ʵķ���
(4)�ȵ�ȼ�ƾ��ƣ�����ˮ�������ٵ�ȼ�ƾ���� ��ֹ�ȵ�ȼ�ƾ���������µ�Fe��O2�ķ�Ӧ
(5)��ʯ�� ��ȥ��Ӧ������H2�е�ˮ����
(6)H2 �鴿 ���Թ��ռ�һ�Թ����壬�����ƾ��ƻ��棬��������������ǡ�ž���ı���������֤����������������(��������)������ǡ��ۡ�����������֤���Ǵ��������� ��ֹ��ȼʱ������������������ը

 ����ѧ����ϵ�д�
����ѧ����ϵ�д�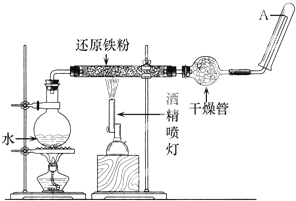 �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ������Ӧ��ʵ�顰��
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ������Ӧ��ʵ�顰�� �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
 �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������Ȳ�ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱����ش��ʵ���е����⣮
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������Ȳ�ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱����ش��ʵ���е����⣮