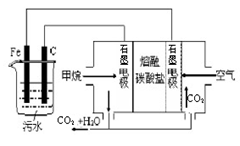
��Ŀ����
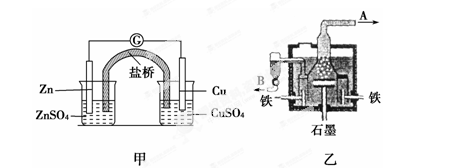
��ͼ�ס����ǵ绯ѧʵ��װ�á��йص缫��Ӧ����������ǣ�

| A�������ձ���ʢ��NaCl��Һ����ʯī���ϵĵ缫��ӦʽΪO2��2H2O��4e��= 4OH�� |
| B�������ձ���ʢ�������е��ܷ�ӦΪ |
| C�������ձ���ʢ��CuSO4��Һ������������ϵĵ缫��ӦʽΪFe��2e��=Fe2�� |
| D������ʼʱ����ʢ��200 mL pH=5��CuSO4��Һ��һ��ʱ�����Һ��pH��Ϊ1����Ҫʹ��Һ�ָ������ǰ��״̬��������Һ�м���0.6 g CuO |
D
���������A�������ձ�����ҺΪ�Ȼ�����Һ�������ڸ�����������ʴ��ʯī���������缫��ӦʽΪO2��2H2O��4e��= 4OH������ȷ��B�������ձ�ʢNaCl��Һ���ݵ��ӵ������֪ʯī���������������������е��ܷ�ӦΪ2Cl����2H2O
 2OH����H2����Cl2������ȷ��C����ʱ������������ȷ��D��һ��ʱ�����Һ��PHΪ1����ʱ��Һ��H+�����ʵ���Ũ��Ϊ0.1mol/L�������ʵ���Ϊ0.2mol,����ˮ������������ӣ�����ܷ�ӦʽΪ2Cu2+��2H2O
2OH����H2����Cl2������ȷ��C����ʱ������������ȷ��D��һ��ʱ�����Һ��PHΪ1����ʱ��Һ��H+�����ʵ���Ũ��Ϊ0.1mol/L�������ʵ���Ϊ0.2mol,����ˮ������������ӣ�����ܷ�ӦʽΪ2Cu2+��2H2O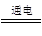 4H+��O2����2Cu��Ҫʹ��Һ�ָ�ԭ״̬���ɼ���CuO��һ��ʱ�����Һ��pH��Ϊ1����c��H+��=0.1mol/L-10-5mol/L=0.1mol/L��n��H+��=0.2L��0.1mol/L=0.02mol�����ɵ�ⷴӦ��֪������Cu�����ʵ���Ϊ0.01mol����Cuԭ���غ��֪��m��CuO��=0.01mol��80g/mol=0.8g������
4H+��O2����2Cu��Ҫʹ��Һ�ָ�ԭ״̬���ɼ���CuO��һ��ʱ�����Һ��pH��Ϊ1����c��H+��=0.1mol/L-10-5mol/L=0.1mol/L��n��H+��=0.2L��0.1mol/L=0.02mol�����ɵ�ⷴӦ��֪������Cu�����ʵ���Ϊ0.01mol����Cuԭ���غ��֪��m��CuO��=0.01mol��80g/mol=0.8g������
��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ
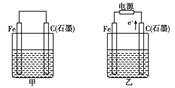
 Fe(OH)2+Ni(OH)2
Fe(OH)2+Ni(OH)2 H2
H2
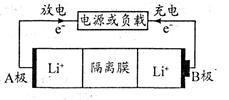
 Li1-xCoO2+LixC6�������й�˵����ȷ����
Li1-xCoO2+LixC6�������й�˵����ȷ����