��Ŀ����
���Ѿ���ȡ�Ȼ��ơ��塢þ�Ȼ�ѧ���ʵĸ���±ˮ�У��������й��������������ʵ⡣����������⣺
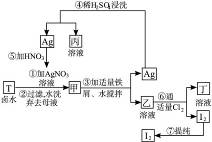
��1��д���ҡ��������ʵĻ�ѧʽ����___________����___________��
��2���ڢܲ���������ϡ�����ϴ��Ŀ����___________��
a.��ȥδ��Ӧ��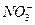
b.��ȥδ��Ӧ��I-
c.��ȥδ��Ӧ��Fe
d.��ȥ��������
��3���ڢ߲������ɹ��ᴿI2�����ַ�����___________��___________����Ҫ��д���岽�裩��
��4��ʵ���Ҽ���I2�ķ�����_________________________________��
��5�������ʼ����ױ�ڣ���ԭ���ǣ��û�ѧ����ʽ��ʾ����______________________��
����ʾ��3Ag+4HNO3====3AgNO3+NO��+2H2O��
(6)������ʵ�������գ�
д���٢ڢ۷�Ӧ�Ļ�ѧ����ʽ��
��____________________________________________________________________;
��____________________________________________________________________;
��____________________________________________________________________��

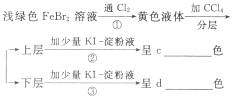
��1��д���ҡ��������ʵĻ�ѧʽ����___________����___________��
��2���ڢܲ���������ϡ�����ϴ��Ŀ����___________��
a.��ȥδ��Ӧ��
b.��ȥδ��Ӧ��I-
c.��ȥδ��Ӧ��Fe
d.��ȥ��������
��3���ڢ߲������ɹ��ᴿI2�����ַ�����___________��___________����Ҫ��д���岽�裩��
��4��ʵ���Ҽ���I2�ķ�����_________________________________��
��5�������ʼ����ױ�ڣ���ԭ���ǣ��û�ѧ����ʽ��ʾ����______________________��
����ʾ��3Ag+4HNO3====3AgNO3+NO��+2H2O��
(6)������ʵ�������գ�

д���٢ڢ۷�Ӧ�Ļ�ѧ����ʽ��
��____________________________________________________________________;
��____________________________________________________________________;
��____________________________________________________________________��
��1��FeI2 FeCl3
(2)C (3)���� ��ȡ
��4����I2���ڵ�����Һ�У���Һ����ɫ
��5��2Ag====I2Ag+I2
(6)c.�� d.��
��2FeBr2+3Cl2====2FeCl3+2Br2
��2FeCl3+2KI====2FeCl2+I2+2KCl
��2KI+Br22====KBr+I2
(2)C (3)���� ��ȡ
��4����I2���ڵ�����Һ�У���Һ����ɫ
��5��2Ag====I2Ag+I2
(6)c.�� d.��
��2FeBr2+3Cl2====2FeCl3+2Br2
��2FeCl3+2KI====2FeCl2+I2+2KCl
��2KI+Br22====KBr+I2
�����ʵ�ת��ͼ�������ۣ��ɵ��������������͵⻯����Ӧ���ɵģ��������ڸ÷�Ӧ��ֻ�ܱ�����Ϊ+2�ۣ�������FeI2,������FeI3,��Cl2��FeI2����ΪI2��FeCl3��3Cl2+2FeI2====2I2+2FeCl3���ڵڢܲ���������ϡ�����ϴ��ʱ���dz�ȥ�������������I2�������������������л��ܼ�����ƾ������Ȼ�̼�ȣ����ᴿI2������������ȡ�ķ���������I2�����۵�ˮ��Һ����ɫ�����Ǽ��鵥��I2�ij��÷�����Ҳ�Ǽ�����۵ij��÷�������AgI���ȶ��Խϲ���������ֽ����ɵ������͵⡣

��ϰ��ϵ�д�
�����Ŀ
 ZnCl����ZnI����ICl+H����
ZnCl����ZnI����ICl+H����