��Ŀ����
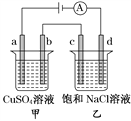
����Ŀ��������ͼ��ʾ����װ�����һ��������ⱥ��ʳ��ˮ���ⶨ���ʱ����������������ͼ��������������Ե�ʵ��װ�á�
��1����ѡ���� ����ʱ�����ӿڵ�˳����(����ӿڵĴ�����ĸ)��A �� �� �� ��B �� �� �� ��_____________
��2��ʵ �� ʱ �� װ �� �� �� ʯ ī �� �� �� �� Դ �� _____�� �� �� �� �� �� �� �� �� Ӧ ʽ Ϊ_____�����缫�ӵ�Դ��_____�����������ĵ缫��ӦʽΪ_____�� �˵���ܷ�Ӧ����ʽΪ_________��
��3��ʵ���ò������������(������ɱ�״��)Ϊ 5.60 mL��������Һ�����ǡ��Ϊ50.0 mL������Һ�� OH����Ũ��Ϊ_____��
���𰸡�GFHDEC��2Cl����2e��===Cl2����2H����2e��=H2��2NaCl��2H2O![]() 2NaOH��H2����Cl2��c(OH��)��0.01 mol��L��1
2NaOH��H2����Cl2��c(OH��)��0.01 mol��L��1
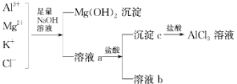
��������
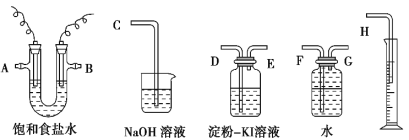
��1��U�ܷ�Ӧ���е������缫δ���ĸ�����������������������ѡ�á�����Ӧ������������Щ������������˳��ȡ����A��BΪ���ֵ缫����缫�����ʵ��Ҫ����A�ϵ缫Ϊ���ʵ缫��B�ϵ缫Ϊʯī�缫����Ӧ��������ѡ�õĸ������ӿ�����˳��ΪA������ƿ��G�D��F����ˮ������Ͳ��H���ܣ�����Ͳ�������ų���ˮ�����Բⶨ���������������B��ϴ��ƿ��D�D��E�����ɵ�������ϴ��ƿ���������۵⻯����Һ����֤���������ԣ����������ͨ���ձ����C���ܣ�������������������������ֹ��Ⱦ�������ʴ�Ϊ��G��F��H��D��E��C����2�����ݵ�ⱥ��ʳ��ˮ�����������ķ�Ӧʽ��2Cl����2e��===Cl2����Ϊ��ֹ�缫����ʴ��ʵ����һ��ѡ��ʯī��������������������ˮ�������H�����ӱ���ԭ��2H����2e��===H2�����Ӷ��ƻ�ˮ�ĵ���ƽ�⣬�������������γ��������ƣ��Լ��ԣ�����ͨ��ʹ�����缫�������ܷ�Ӧ����ʽΪ��2NaCl��2H2O![]() 2NaOH��H2����Cl2�����ʴ�Ϊ������2Cl����2e��===Cl2��������2H����2e��===H2����2NaCl��2H2O
2NaOH��H2����Cl2�����ʴ�Ϊ������2Cl����2e��===Cl2��������2H����2e��===H2����2NaCl��2H2O![]() 2NaOH��H2����Cl2������3��
2NaOH��H2����Cl2������3��
��֪����������5.60 mL�������ʵ���Ϊ![]() ��2��5��10��4mol���ɷ���ʽ�ɵ�
��2��5��10��4mol���ɷ���ʽ�ɵ�
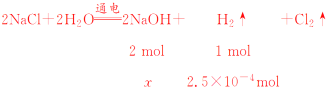
x��2��2.5��10��4mol��5��10��4mol
c(OH��)��![]() ��0.01 mol��L��1���ʴ�Ϊ��0.01 mol��L��1��
��0.01 mol��L��1���ʴ�Ϊ��0.01 mol��L��1��

����Ŀ��ijʵ��С����100 mL 0.50 mol��L��1 NaOH��Һ��100 mL 0.55 mol��L��1��������к��ȵIJⶨ��װ����ͼ��ʾ���ش��������⣺
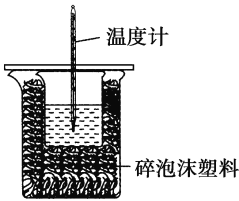
��1����ʵ�鹲��Ҫ400 mL NaOH��Һ��ʵ���������Ƹ���Һʱ������Ҫ����NaOH����______g��
��2��ͼ��װ��ȱ�ٵ�������______________________________��
��3�������Թ�����ԭ����________________________________��
��4������ĭ���ϼ���ĭ���ϰ��������___________________��
��5�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к���(��H)________(����ƫ������ƫС������������)����ԭ����_______��
��6������д�±��е�ƽ���¶Ȳ
ʵ����� | ��ʼ�¶�T1/�� | ��ֹ�¶� T2/�� | ƽ���¶Ȳ� (T2��T1)/�� | |||
HCl | NaOH | ƽ��ֵ | ||||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ________ | |
2 | 27.0 | 27.4 | 27.2 | 33.3 | ||
3 | 25.9 | 25.9 | 25.9 | 29.8 | ||
4 | 26.4 | 26.2 | 26.3 | 30.4 | ||
��7������ø÷�Ӧ�ų�������Ϊ2.865 kJ����д��������NaOH��Һ��Ӧ���к��ȵķ���ʽ��__________________________________________��