��Ŀ����
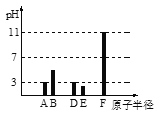
����Ŀ��25��ʱ��������Һ��pH������
�� | �� | �� | �� | |
pH | 3 | 3 | 11 | 11 |
��Һ | ���� | ���� | NaOH��Һ | ��ˮ |
��1��������Һ����ˮ�����c(H+)ˮ��c(OH-)ˮ��______________(mol/L)2��
��2�����м������������ƾ������Һ��pH________��������������������С��������������
��3������������Һ����������������Һ��c(Na��)______c(CH3COO��)������������������������������
��4����һ������Ģٺ͢�ǡ����ȫ��Ӧ�����Ȼ����Һ������Һ������Ũ���ɴ�С��˳����_________________________________��
��5��25��ʱ��a mol/L������Һ��b mol/LNaOH��Һ�������Ϻ�pH��7�������ĵ��볣��Ka��__________�����ú�a��b�Ĵ���ʽ��ʾ��
���𰸡�10-22 ���� �� c(Cl��)��c(NH4+)��c(H+)��c(OH��) ![]()
��������
��1���ȼ���pH=11��NaOH��Һ����ˮ�����c(H+)ˮ������c(H+)ˮ=c(OH-)ˮ����c(H+)ˮ��c(OH-)ˮ��
��2��������Һ�м�������ƾ��壬ʹ���������Ũ������ϴ���ĵ���ƽ�����pH�仯��
��3��pH=11��NaOH��Һ��pH=3�Ĵ�����Һ�������ϣ�����������ݵ���غ����������Һ��c(Na��)��c(CH3COO��)�Ĺ�ϵ��
��4���Ȼ����Һ�д���Cl����NH4+��H+��OH�������NH4+��ˮ��ƽ���������Һ������Ũ�ȴ�С��ϵ��
��5������ĵ��볣��Ka��c(CH3COO��)��c(H+)/ c(CH3COOH)���ʼ����c(CH3COO��)��c(H+)�� c(CH3COOH)������㼴����
��1��pH=11��NaOH��Һ��c(OH-)=10-3mol/L��25��ʱ����ˮ�����c(H+)ˮ��10-11mol/L��c(H+)ˮ=c(OH-)ˮ��10-11mol/L����c(H+)ˮ��c(OH-)ˮ��10-22 mol2/L2��
��2��������Һ�м�������ƾ��壬ʹ���������Ũ��������ĵ���ƽ�������ƶ�����Һ���Լ�����pH����
��3��NaOHΪǿ�pH=11��NaOH��Һ��c(NaOH)=10-3mol/L������Ϊ���ᣬpH=3�Ĵ�����Һ��c(CH3COOH)>10-3mol/L������Һ�������ϣ����������Һ��������������Һ��c(Na��)<c(CH3COO��)��
��4�����Ȼ����Һ�д���Cl����NH4+��H+��OH�����ɵ���غ��֪c(Cl��)+c(OH��) =c(NH4+)+c(H+)�����ڴ���NH4++H2ONH3��H2O+H+������Һ�����ԡ������Һ������Ũ���ɴ�С��˳����c(Cl��)��c(NH4+)��c(H+)��c(OH��)��
��5����������������1:1��Ӧ��õ������ƣ���Һ�Լ��ԡ�a mol/L������Һ��b mol/LNaOH��Һ�������Ϻ���Һ�����ԣ��������ʣ�࣬�ʻ����Һ�мȺ��д����ƣ��ֺ��д��ᡣ���ڵ���غ�c(CH3COO��) +c(OH��)=c(Na+)+c(H+),c(CH3COO��)= c(Na+)=b/2 mol/L����c(CH3COOH)=(a-b)/2 mol/L������ĵ��볣��Ka��c(CH3COO��)��c(H+)/ c(CH3COOH)= b/2��10-7/(a-b)/2=b/(a-b)��10-7��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ����

�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ���������ܵĽ�ˮ��Ϊ___________���a����b������
��2���ü�װ����������MnO4-����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽___________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ��ٴٽ�����������Ӧ����_______________________________��
��4�����ȱ����װ�ã����ܲ����ĺ����___________________����װ�õ�������__________________��
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2 ����_______________�����ţ���
a.FeCl3��Һ������KSCN�� b.H2O2��Һ C.��ˮ d.AgNO3��Һ
��6����Ӧ����ȥ����1.19g����Ӧ������װ�õ��Թ����ռ���2.38 gSnCl4����SnCl4�IJ���Ϊ____________________��