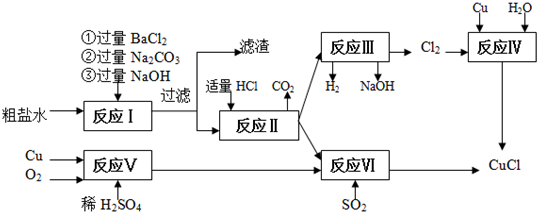
��Ŀ����
8���������ƾ��壨CaO2•8H2O���ʰ�ɫ������ˮ��������350�����ҿ�ʼ�ֽ�ų��������������ƿ����ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ��Ӧ�������ȣ�ʵ���ҿ��ù�ҵ̼��ƣ���MgCO3��FeCO3�����ʣ���ȡ������̼��ƣ�Ȼ�����ô���̼�����ȡ�������ƣ�����Ҫ������ͼ1��
�ش��������⣺
��1�������������漰�Ļ�ѧ��Ӧ����������ԭ��Ӧ����1������д����������һ�������ӷ���ʽ��3FeCO3+10H++NO3-=3Fe3++NO��+3CO2��+5H2O��
��2����Ӧ������CaO2•8H2O�Ļ�ѧ��Ӧ����ʽΪCaCl2+H2O2+2NH3+8H2O=CaO2•8H2O+2NH4Cl����Ӧʱ�ñ�ˮ��ȴ����Ҫԭ���Ƿ�ֹH2O2�ֽ⣬����������ʻ�CaO2•8H2O�ܽ�ȣ���߲��ʣ�
��3�����������ƾ����������м��Ȳ��������¶ȣ������Ʒ�������¶ȵı仯��ͼ2������ʾ����350���Ժ����ù������ʵĻ�ѧʽΪCaO��
���� ̼��ơ�̼��þ��ϡ���ᷴӦ����������ơ�����þ�������̼��̼��������ϡ���ᷴӦ������������������̼��NO����Һ�м���Ũ��ˮ��þ���ӡ�������ת��Ϊ������þ�������������������˳�ȥ����Һ�м���̼��什������ת��Ϊ̼��ƣ����˷���õ�̼��ƣ��������ᷴӦ�����Ȼ��ƣ��ڼ���������⡢�����õ���Ӧ�õ�CaO2����Һ��ȴ�ᾧ�õ�CaO2•8H2O������Ʒ�к����Ȼ�泥�
��1����Ӧ����ֻ��FeCO3�����ᷴӦ��������ԭ��Ӧ��
��2���÷�Ӧ�ķ�Ӧ��ΪCaCl2��H2O2��NH3����Ӧ����CaO2•8H2O��NH4Cl��
H2O2�����ֽ⣬���ñ�ˮ�����¶���0�����ң���ֹ��ֽ⣬��������ʣ�ͬʱ�¶ȵ��ܽϵ��ܽ�ȣ���߲��ʣ�
��3��������2.16g���ȵ�350���Ϊ0.56g���ڼ��ȹ����й����и�Ԫ�ص������䣬�ݴ˼���ʣ�������CaԪ�����������������غ����ʣ�������OԪ������������ȷ����ѧʽ��
��� �⣺̼��ơ�̼��þ��ϡ���ᷴӦ����������ơ�����þ�������̼��̼��������ϡ���ᷴӦ������������������̼��NO����Һ�м���Ũ��ˮ��þ���ӡ�������ת��Ϊ������þ�������������������˳�ȥ����Һ�м���̼��什������ת��Ϊ̼��ƣ����˷���õ�̼��ƣ��������ᷴӦ�����Ȼ��ƣ��ڼ���������⡢�����õ���Ӧ�õ�CaO2����Һ��ȴ�ᾧ�õ�CaO2•8H2O������Ʒ�к����Ȼ�泥�
��1����Ӧ����ֻ��FeCO3�����ᷴӦ��������ԭ��Ӧ����Ӧ����ʽΪ��3FeCO3+10H++NO3-=3Fe3++NO��+3CO2��+5H2O��
�ʴ�Ϊ��1��3FeCO3+10H++NO3-=3Fe3++NO��+3CO2��+5H2O��
��2���÷�Ӧ�ķ�Ӧ��ΪCaCl2��H2O2��NH3����Ӧ����CaO2•8H2O��NH4Cl����Ӧ����ʽΪ��CaCl2+H2O2+2NH3+8H2O=CaO2•8H2O+2NH4Cl��
H2O2�����ֽ⣬���ñ�ˮ�����¶���0�����ң���ֹ��ֽ⣬��������ʣ�ͬʱ�¶ȵ��ܽϵ��ܽ�ȣ���߲��ʣ�
�ʴ�Ϊ��CaCl2+H2O2+2NH3+8H2O=CaO2•8H2O+2NH4Cl����ֹH2O2�ֽ⣬����������ʻ�CaO2•8H2O�ܽ�ȣ���߲��ʣ�
��3��������2.16g���ȵ�350���Ϊ0.56g���ڼ��ȹ����й����и�Ԫ�ص������䣬ʣ�������n��Ca��=n��CaO2•8H2O��=$\frac{2.16g}{216g/mol}$=0.01mol��m��Ca��=0.01mol��40g/mol=0.4g����ʣ�������m��O��=0.56g-0.4g=0.16mol����n��O��=$\frac{0.16mol}{16g/mol}$=0.01mol����n��Ca����n��O��=1��1��ʣ�����Ļ�ѧʽΪCaO��
�ʴ�Ϊ��CaO��
���� ���⿼�黯ʵ���Ʊ��������̡���Ӧԭ����������ԭ��Ӧ�ζ�Ӧ�á��й�ͼ��ļ�����й����⣬�Ƕ�ѧ���ۺ������Ŀ��飬�Ѷ��еȣ�

| A�� | ������ | B�� | �춡�� | C�� | ������ | D�� | ������ |
| A�� | c��H+��=0.1mol/L����Һ��Na+��NH4+��SO42-��S2O32- | |
| B�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��10-12����Һ��K+��AlO2-��CO32-��Na+ | |
| C�� | c��Fe2+��=0.1mol/L����Һ��H+��Al3+��NO3-��SCN- | |
| D�� | ����ˮ�������c��H+��=1��10-12mol/L����Һ��Fe3+��ClO-��Na+��SO42- |
-CH3+2KMnO4$\stackrel{��}{��}$-COOK+KOH+2MnO2��+H2O
-COOK+HCl��-COOH+KCl
ʵ�鷽����һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
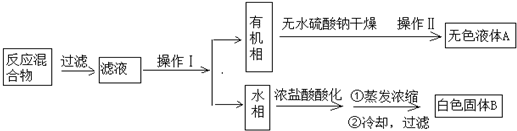
��֪����������Է�������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����������л���һ�㶼�й̶��۵㣮
��1��������Ϊ��Һ��������Ϊ����
��2����ɫҺ��A�Ǽױ������Լ���A���Լ�������KMnO4��Һ����������Һ��ɫ��ȥ��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ������ɱ������ݣ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У� �����ܽ⣬��ȴ�ᾧ������ | �õ���ɫ�������ɫ��Һ | �� |
| �� | ȡ������Һ���Թ��У� ����ϡHNO3��AgNO3��Һ | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ���壬�ⶨ�۵� | ��ɫ������122.4��������ȫ�ۻ� | ��ɫ�����DZ����� |
| A�� | W ��Y �����γ����ӻ����� | |
| B�� | X �ļ��⻯����ͬ����Ԫ�ص��⻯���зе���ߣ�˵��X �ķǽ�������ǿ | |
| C�� | X ��Z �γɵ�ԭ�Ӹ���l��1 �Ļ������У��Ⱥ��м��Լ��ֺ��зǼ��Լ� | |
| D�� | ��ҵ�����У�ͨ���õ��Z ����������������ȡZ �ĵ��� |