��Ŀ����
����Ŀ��ij����С������ͼװ�ý���ʵ�飬�Իش�����������

��1������ʼʱ����K��a���ӣ���B���ĵ缫��ӦʽΪ______________��
��2������ʼʱ����K��b���ӣ���B���ĵ缫��ӦʽΪ__________���ܷ�Ӧ�����ӷ���ʽΪ____________��
��3������ʼʱ����K��b����.����˵����ȷ����_______��
A.��Һ��Na����A���ƶ�
B.��A�����ݳ���������ʹʪ���KI������ֽ����
C.��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D.����״����B������2.24 L���壬����Һ��ת��0.2 mol����
��4����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼװ�õ���������Һ����ȡ������������������������ء�
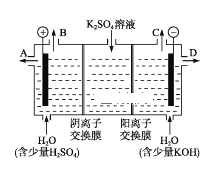
���õ��۵�������ӦʽΪ________��
���Ƶõ�����������Һ�ӳ���(����A������B������C���� ��D��)________������
��������������������������ǿ����ƽ���ƶ�ԭ������ԭ��_________________________��
���𰸡�Fe-2e- = Fe2�� 2H��+2e- = H2�� 2Cl-+2H2O=2OH��+ H2��+ Cl2�� B 2H2O��4e- =4H�� +O2������4OH�D�D4e����2H2O��O2���� D H2O![]() H��+ OH����H�������������ŵ磬����ˮ�ĵ���ƽ�������ƶ���ʹc(OH��)>c(H��)����Һ�Լ���
H��+ OH����H�������������ŵ磬����ˮ�ĵ���ƽ�������ƶ���ʹc(OH��)>c(H��)����Һ�Լ���
��������
(1)��ʼʱ����K��a�����γ�ԭ��ط�Ӧ��B�缫��������ʧ���������������ӣ��缫��ӦΪ��Fe-2e-=Fe2+���ʴ�Ϊ��Fe-2e-=Fe2+��
(2)����K��b���ӣ�װ��Ϊ���أ���Ϊ������������ԭ��Ӧ�������ӵõ�����������������B�缫��ӦΪ2H++2e-=H2������ⱥ��ʳ��ˮ�����������ơ�����������������ܷ�Ӧ�����ӷ���ʽΪ2Cl-+2H2O ![]() 2OH-+H2��+Cl2�����ʴ�Ϊ��2H++2e-=H2����2Cl-+2H2O
2OH-+H2��+Cl2�����ʴ�Ϊ��2H++2e-=H2����2Cl-+2H2O ![]() 2OH-+H2��+Cl2����
2OH-+H2��+Cl2����
(3)A�����������������������ƶ���BΪ��������Һ��Na+��B���ƶ�����A����B��A�������������������ܹ��û����⻯����Һ�еĵ����ɵⵥ�ʣ��������۱�������B��ȷ��C����Ӧһ��ʱ��������HCl���壬�ɻָ������ǰ����ʵ�Ũ�ȣ����Ǽ������ᣬ��C����D������״����B������2.24L���������ʵ���Ϊ0.1mol�����ݵ缫��Ӧʽ2H++2e-=H2��֪����·��ת��0.2mol���ӣ������Ӳ��ܾ�����Һ����D���ʴ�Ϊ��B��
(4)�ٵ��ʱ��������ʧ���ӷ���������Ӧ����Һ�е����������ӵķŵ�����������������ӣ���������������������ʧ��������ˮ��������������ҺΪ���ᣬ�ʵ缫��ӦʽΪ2H2O��4e- =4H�� +O2��(��4OH�D�D4e����2H2O��O2��)���ʴ�Ϊ��2H2O��4e- =4H�� +O2��(��4OH�D�D4e����2H2O��O2��)��
���������������ӷŵ磬�������������������ƶ������������ӷŵ磬��˼������������ƶ����������������������������ɣ���������������Һ�ӳ���D�������ʴ�Ϊ��D��
�۵���������������ˮ������������ӷŵ磬2H++2e-=H2��������ˮ�ĵ���ƽ�������ƶ�����������������Ũ�ȴ��������ӣ���Һ��ʾ���ԣ��ʴ�Ϊ��H2O![]() H��+ OH����H�������������ŵ磬����ˮ�ĵ���ƽ�������ƶ���ʹc(OH��)>c(H��)����Һ��ʾ������
H��+ OH����H�������������ŵ磬����ˮ�ĵ���ƽ�������ƶ���ʹc(OH��)>c(H��)����Һ��ʾ������

����Ŀ�����и�������У������ԭ��ص���( )
A | B | C | D | |
�������� | ZnƬ��CuƬ | CuƬ�� CuƬ | ZnƬ��CuƬ | ZnƬ��CuƬ |
������Һ | ϡH2SO4 | NaCl��Һ | ֲ���� | �ƾ� |
A. AB. BC. CD. D





