��Ŀ����
��4�֣����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.1000mol��L-1HCl����Һ�����к͵ζ����÷�̪��ָʾ������
��ش��������⣺
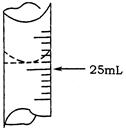
��1������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ ��
��2����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
ѡȡ�����������ݣ����������NaOH��Һ�����ʵ���Ũ��Ϊ ��С���������λ����
��3��������Щ������ʹ�ⶨ���ƫ�� ������ţ���
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
�� Fe2O3(s)+3CO(g)="=" 2Fe(s)+3CO2(g) ��H�� �D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H�� �D47.2 kJ?mol-1
�� Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
__________________________________________________��
��ش��������⣺
��1������ѧ����ʵ������У���¼�ζ�ǰ�ζ�����Һ�����Ϊ1.10mL���ζ���Һ����ͼ�����ʱ���ı���Һ�����Ϊ ��

��2����ѧ����������ƽ��ʵ�飬���ݼ�¼���£�
| ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol��L-1HCl��Һ�����/mL | |
| �ζ�ǰ�̶� | �ζ���̶� | ||
| 1 | 25.00 | 0.00 | 26.11 |
| 2 | 25.00 | 1.56 | 31.30 |
| 3 | 25.00 | 0.22 | 26.31 |
��3��������Щ������ʹ�ⶨ���ƫ�� ������ţ���
A����ƿ������ˮϴ�������ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ�������ñ�Һ��ϴ
C���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ζ���������ʧ
D���ζ�ǰ������ȷ���ζ����ӵζ��ܶ���
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
�� Fe2O3(s)+3CO(g)="=" 2Fe(s)+3CO2(g) ��H�� �D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) ��H�� �D47.2 kJ?mol-1
�� Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
__________________________________________________��
��1��23.80mL
��2��0.1092mol��L-1
��3��AC
��4��CO(g)+FeO(s)=Fe(s)+CO2(g) ��H��-218.0kJ?mol-1
��2��0.1092mol��L-1
��3��AC
��4��CO(g)+FeO(s)=Fe(s)+CO2(g) ��H��-218.0kJ?mol-1
�����������1��ͼ�еζ�������ʾ�Ŀ̶�Ϊ23.80mL�������ĵ����Ϊ23.80-1.10=23.80mL��
��2�����ĵ������ƽ�����Ϊ
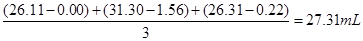
����Һ��Ũ��Ϊ
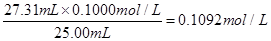
(3)��ƿ����Ҫ��ϴ���ζ�ǰ��ʽ�ζ��ܼ������δ�ų����ᵼ��ʵ��������С���Ӷ��������ս��ƫ�ߡ�
(4)CO(g)+FeO(s)=Fe(s)+CO2(g),�䷴Ӧ��
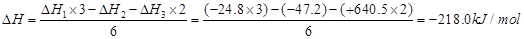
�����Ȼ�ѧ����ʽΪCO(g)+FeO(s)=Fe(s)+CO2(g) ��H��-218.0kJ?mol-1
�������ڽ����ʱ�������к͵ζ���Ӧע�����ζ��ܵ�ʹ�ã���ʽ�ζ��ܲ���װ�������Լ������⣬�����Ȼ�ѧ����ʽ����д�����ѵ����ڷ�Ӧ�ȵļ��㡣���㷴Ӧ��ʱ��Ӧ���ø�˹���ɣ���������ʽʽ���мӼ��˳����õ�Ŀ�귽��ʽ����Ӧ�ķ�Ӧ��Ҳ���мӼ��˳������ɵõ�Ŀ�귽��ʽ�ķ�Ӧ�ȡ�

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ