��Ŀ����
������Һ���й����ʵ���Ũ�ȹ�ϵ��ȷ����
A��pH��ȵ�CH3COONa��NaOH��Na2CO3������Һ��
c(NaOH) < c(CH3COONa) < c(Na2CO3)
B����֪0.1 mol?L��1 ��Ԫ��H2A��Һ��pH��4������0.1 mol?L��1 Na2A��Һ�У�
c(OH��) �� c(HA��) + c(H��) + 2c(H2A)
C����0.1 mol?L��1������Һ��ˮϡ�ͣ�����Һ�е�c(H��)��c(OH��)����С
D����0.1 mol?L��1�İ�ˮ�м�����������粒��壬����Һ��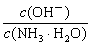 ����
����
B

��ϰ��ϵ�д�
�����Ŀ
������Һ���й����ʵ�Ũ�ȹ�ϵ��ȷ���ǣ�������
| A��c��NH4+����ȵģ�NH4��2SO4��NH4HSO4��NH4Cl��Һ�У�c��NH4HSO4����c[��NH4��2SO4]��c��NH4Cl�� | B�����������Һ�м����������ᣬ�õ������Ի����Һ��c��Na+����c��CH3COO-����c��H+����c��OH-�� | C��1.0 mol/L Na2CO3��Һ��c��OH-��=c��HCO3-��+c��H+��+2c��H2CO3�� | D��ij��Ԫ�������ʽ��NaHA��Һ�У�c��H+��+c��Na+��=c��OH-��+c��HA-��+c��A2-�� |