ĢāÄæÄŚČŻ
”¾ĢāÄæ”æŅŃÖŖA”¢B”¢C”¢D”¢E”¢F¶¼ŹĒ¶ĢÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪµŻŌö”£AŌ×ӵĵē×Ó²ćŹżÓėĖüµÄŗĖĶāµē×Ó×ÜŹżĻąĶ¬£¬¶ųBŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµÄ2±¶£¬CµÄĒā»ÆĪļæÉŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬BŗĶDæÉŅŌŠĪ³ÉĮ½ÖÖĘųĢ¬»ÆŗĻĪļ£¬EŌ×ÓŗĖĶāµē×Ó×ÜŹż±ČBµÄ2±¶ÉŁ1£¬FŌŚ±¾ÖÜĘŚŌŖĖŲÖŠŌ×Ó°ė¾¶×īŠ””£Ōņ£ŗ
(1)A”¢B”¢C”¢D”¢E”¢FµÄĆū³Ę·Ö±šŹĒ _____________________________ ”£
(2)ŌŚAÖĮFÖŠČĪŃ”ŌŖĖŲ£¬Š“³öŅ»ÖÖŗ¬·Ē¼«ŠŌ¼üµÄĄė×Ó»ÆŗĻĪļµÄµē×ÓŹ½ _________ ”£
(3)ÓÉBŗĶD×é³É£¬ĒŅBŗĶDµÄÖŹĮæ±ČĪŖ3”Ć8µÄ»ÆŗĻĪļµÄµē×ÓŹ½ŹĒ _________ £¬øĆĪļÖŹÓėEµÄĶ¬ÖÜĘŚĻąĮŚÖ÷×åŌŖĖŲµÄµ„ÖŹ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ__________________ ”£
(4)Fµ„ÖŹÓėEµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________”£
(5)½«8g BA4ĶźČ«Č¼ÉÕŗó»Öø“µ½ŹŅĪĀ£¬·Å³öČČĮæa kJ£¬Š“³ö±ķŹ¾BA4µÄČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½_______”£
”¾“š°ø”æĒā”¢Ģ¼”¢µŖ”¢Ńõ”¢ÄĘ”¢ĀČ ![]()
![]() CO2+2Mg
CO2+2Mg![]() C+2MgO 2NaOH+Cl2=NaCl+NaClO+H2O CH4(g)+2O2(g)=CO2(g)+2H2O(l)”÷H=-2akJ/mol
C+2MgO 2NaOH+Cl2=NaCl+NaClO+H2O CH4(g)+2O2(g)=CO2(g)+2H2O(l)”÷H=-2akJ/mol
”¾½āĪö”æ
AŌ×ӵĵē×Ó²ćŹżÓėĖüµÄŗĖĶāµē×Ó×ÜŹżĻąĶ¬£¬Ó¦ĪŖHŌŖĖŲ£»BŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒ“ĪĶā²ćµÄ2±¶£¬Ó¦ĪŖCŌŖĖŲ£»CµÄĒā»ÆĪļæÉŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬Ó¦ĪŖNŌŖĖŲ£¬ŠĪ³ÉµÄĒā»ÆĪļĪŖ°±Ęų£¬ĪŖ¼īŠŌĘųĢ壻BŗĶDæÉŅŌŠĪ³ÉĮ½ÖÖĘųĢ¬»ÆŗĻĪļ£¬Ó¦·Ö±šĪŖCOŗĶCO2£¬ŌņDĪŖOŌŖĖŲ£»EŌ×ÓŗĖĶāµē×Ó×ÜŹż±ČBµÄ2±¶ÉŁ1£¬ŌņEµÄŌ×ÓŠņŹżĪŖ11£¬Ó¦ĪŖNaŌŖĖŲ£»A”¢B”¢C”¢D”¢E”¢F¶¼ŹĒ¶ĢÖÜĘŚµÄŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪµŻŌö£¬ĒŅFŌŚ±¾ÖÜĘŚŌŖĖŲÖŠŌ×Ó°ė¾¶×īŠ”£¬Ó¦ĪŖClŌŖĖŲ”£
(1) ×ŪÉĻĖłŹöA”¢B”¢C”¢D”¢E”¢FµÄĆū³Ę·Ö±šŹĒĒā”¢Ģ¼”¢µŖ”¢Ńõ”¢ÄĘ”¢ĀČ£¬Ņņ“Ė“š°øŹĒ:Ēā”¢Ģ¼”¢µŖ”¢Ńõ”¢ÄĘ”¢ĀČ£»
(2) ŌŚAÖĮFŌŖĖŲÖŠŗ¬·Ē¼«ŠŌ¼üµÄĄė×Ó»ÆŗĻĪļNa2O2£¬Ęäµē×ÓŹ½![]() £¬Ņņ“Ė“š°øŹĒ:
£¬Ņņ“Ė“š°øŹĒ: ![]() ”£
ӣ
(3) BĪŖC”¢DĪŖO£¬ÓÉCŗĶO×é³ÉµÄĒŅCŗĶOµÄÖŹĮæ±ČĪŖ3”Ć8µÄ»ÆŗĻĪļĪŖCO2£¬Ęäµē×ÓŹ½ĪŖ![]() £¬øĆĪļÖŹÓėEĪŖNaµÄĶ¬ÖÜĘŚĻąĮŚÖ÷×åŌŖĖŲMgµÄµ„ÖŹ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCO2+2Mg
£¬øĆĪļÖŹÓėEĪŖNaµÄĶ¬ÖÜĘŚĻąĮŚÖ÷×åŌŖĖŲMgµÄµ„ÖŹ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCO2+2Mg![]() C+2MgO,Ņņ“Ė“š°øŹĒ
C+2MgO,Ņņ“Ė“š°øŹĒ![]() £»CO2+2Mg
£»CO2+2Mg![]() C+2MgO”£
C+2MgOӣ
(4) Fµ„ÖŹĪŖCl2ÓėNaµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2NaOH+Cl2=NaCl+NaClO+H2O£»Ņņ“Ė“š°øŹĒ: 2NaOH+Cl2=NaCl+NaClO+H2O £»
(5) BA4ĪŖCH4£¬½«8g µÄCH4ĶźČ«Č¼ÉÕŗó»Öø“µ½ŹŅĪĀ£¬·Å³öČČĮæa kJ£¬Ōņ1mol CH4ĶźČ«Č¼Éշųö2akJµÄČČĮ棬ŌņCH4µÄČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½CH4(g)+2O2(g)=CO2(g)+2H2O(l)”÷H=-2akJ/mol£¬“š°ø£ŗCH4(g)+2O2(g)=CO2(g)+2H2O(l)”÷H=-2akJ/mol”£

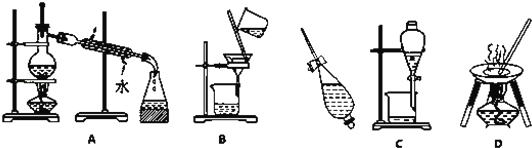
”¾ĢāÄæ”æPOCl3³£ÓĆ×÷°ėµ¼Ģå²ōŌÓ¼Į¼°¹āµ¼ĻĖĪ¬ŌĮĻ£¬ŹµŃéŹŅ²ÉÓĆŃõĘųŃõ»ÆŅŗĢ¬PCl3·ØÖĘČ”POCl3£¬ÓŠ¹ŲĪļÖŹµÄ²æ·ÖŠŌÖŹČēĻĀ±ķ
ĪļÖŹ | ČŪµć/”ę | ·Šµć/”ę | Ļą¶Ō·Ö×ÓÖŹĮæ | ĘäĖū |
PCl3 | -112.0 | 76.0£ØŅ×»Ó·¢£© | 137.5 | Į½Õß»„ČÜ£¬¾łĪŖĪŽÉ«ŅŗĢ壬ÓöĖ®¾ł¾ēĮŅ·“Ӧɜ³Éŗ¬ŃõĖįŗĶĀČ»ÆĒā |
POC13 | 2.0 | 106.0 | 153.5 |
ŹµŃé×°ÖĆ£Ø¼ÓČČ¼°¼Š³ÖŅĒĘ÷ĀŌ£©ČēĻĀ£ŗ

£Ø1£©¼××°ÖƵÄĆū³ĘŹĒ____________£¬øÉŌļ¹ÜµÄ×÷ÓĆŹĒ____________
£Ø2£©×°ÖĆCÖŠÉś³ÉPOCl3µÄ»Æѧ·½³ĢŹ½ĪŖ______________
£Ø3£©×°ÖĆBµÄ×÷ÓĆ³ż¹Ū²ģĘųĢåµÄĮ÷ĖŁĶā£¬»¹ÓŠ________ ”¢________
£Ø4£©·“Ó¦ĪĀ¶Č²»Äܹżøߣ¬ŌŅņŹĒ______________”£
£Ø5£©·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬓żČż¾±ĘæÖŠµÄŅŗĢåĄäČ“ÖĮŹŅĪĀ£¬×¼Č·³ĘČ”29.1g²śĘ·£Ø½öŗ¬PCl3ŌÓÖŹ£©£¬ÖĆÓŚŹ¢ÓŠ60.00 mLÕōĮóĖ®µÄĆܱÕĖ®½āĘæÖŠŅ”¶ÆÖĮĶźČ«ÓėĖ®·“Ó¦£¬½«Ė®½āŅŗÅä³É100.00 mLČÜŅŗ£¬¼ÓČėAgNO3ČÜŅŗÖĮĒ”ŗĆĶźČ«·“Ó¦£¬²āµĆÉś³ÉµÄAgClµÄÖŹĮæĪŖ86.1g£¬Ōņ²śĘ·ÖŠPOC13µÄÖŹĮæ·ÖŹżĪŖ___£Ø±£ĮōČżĪ»ÓŠŠ§Źż×Ö£©