��Ŀ����
����Ŀ���������ȣ�ClO2���Ǽ�������ˮ�Ҳ���ˮ������ѧ��Ӧ�Ļ���ɫ���壬�е�Ϊ11�棬�����ڴ��������ˮ��ijС����ʵ������̽��ClO2��Na2S�ķ�Ӧ����ش��������⣺
��1��ClO2���Ʊ�����֪��SO2+2NaClO3+H2SO4=2ClO2+2NaHSO4��

��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
�����ռ�һ������ClO2��ѡ����ͼ�е�װ�ã�������˳��Ϊa______��������������Сд��ĸ��ʾ����
��2��ClO2��Na2S�ķ�Ӧ

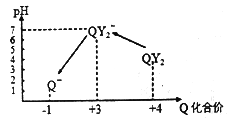
�������ռ�����ClO2��N2ϡ������ǿ���ȶ��ԣ�����������ϡ�ͺ��ClO2ͨ����ͼ��ʾװ���г�ַ�Ӧ���õ���ɫ������Һ��һ��ʱ���ͨ������ʵ��̽�����з�Ӧ�IJ��__

��ClO2��Na2S�ķ�Ӧ�����ӷ���ʽΪ__________�����ڴ��������ˮʱ��ClO2�����Cl2���ŵ���________����дһ������
���𰸡�Cu+2H2SO4��Ũ����CuSO4+SO2��+2H2O g��h��b��c��e��f��d ��SO2���а�ɫ���� �ۼ�������ϡ�����ữ��AgNO3��Һ 8ClO2+5S2-+4H2O=8Cl-+5SO42-+8H+ ClO2����Ч�����ף���������������ȶ���ClO2��ˮ�е��ܽ�ȴ�ʣ���ClO2�������������Ⱦ��������ԭΪCl-ʱ���������ȵõ��ĵ�������������2.5�����𰸺������ɣ�
��������
�������ʵ��Ʊ����ռ����̷�����𣻸������ʵ�ת����д���Ӽ���ѧ��Ӧ����ʽ��
(1)��װ�� A ��Cu��Ũ���ᷴӦ�Ʊ���������ѧ����ʽΪ��Cu+2H2SO4��Ũ��![]()
CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
�ڶ��������a����װ��B�з�Ӧ��Ϊ��ֹ��������Ӧ��B֮ǰ�а�ȫƿ����a��g��h��Ϊ��Ӧ��֣�������Bװ���е�b���������ȷе�ϵͣ���D�н��б�ˮԡ�ռ���Ϊ�˳����ȴ�������ռ�������e�������������������δ��Ӧ��Ķ�������ֹ��Ⱦ����������˳��Ϊ��a��g��h��b��c��e��f��d��
�ʴ�Ϊ��g��h��b��c��e��f��d��
(2)��������ϡ�ͺ�� ClO2 ͨ����ͼ��ʾװ���г�ַ�Ӧ���õ���ɫ������Һ����ȡ����I����Һ���Թܼ��У��μ�Ʒ����Һ�����ᣬƷ��ʼ�ղ���ɫ��˵����Һ����SO2���ɣ�
�ʴ�Ϊ��SO2��
����ȡ����I����Һ���Թ����У�����Ba��OH��2��Һ��������Ϊ��SO42-���ɣ�������뱵���ӷ�Ӧ�������ᱵ��ɫ������
�ʴ�Ϊ����ɫ������
�۽���Ϊ��Cl-���ɣ��а�ɫ�������ɣ��Ȼ���Ϊ��������İ�ɫ���������������ӵIJ���Ϊ���������Թ����еμ�Ba��OH��2��Һ�����������ã�ȡ�ϲ���Һ���Թܱ��ڼ�������ϡ�����ữ����������Һ��
�ʴ�Ϊ����������ϡ�����ữ����������Һ��
��������������֪ClO2 �� Na2S ��Ӧ�������ӡ�������������ɣ����������ӷ���ʽΪ��8ClO2+5S2-+4H2O=8Cl-+5SO42-+8H+�����ڴ��������ˮʱ��ClO2�����Cl2���ŵ���ClO2����Ч�����ף���������������ȶ��� ClO2��ˮ�е��ܽ�ȴ�ʣ���ClO2�������������Ⱦ��������ԭΪCl-ʱ���������ȵõ��ĵ�������������2.5����
�ʴ�Ϊ��8ClO2+5S2-+4H2O=8Cl-+5SO42-+8H+��ClO2����Ч�����ף���������������ȶ��� ClO2��ˮ�е��ܽ�ȴ�ʣ���ClO2�������������Ⱦ��������ԭΪCl-ʱ���������ȵõ��ĵ�������������2.5����

 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�����Ŀ��50mL0.50 mol��L-1��������50mL0.55 mol��L-1��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ʵ���������±���
ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | |
���� | NaOH��Һ | �����Һ | |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |

������Ϊ0.50 mol��L��1NaOH��Һ��0.50 mol��L��1HCl��Һ���ܶȶ���1g��cm��3���кͺ�������Һ�ı�����c��4.18 J��g��1������1��
��ش��������⣺��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������________���ձ�����������ĭ���ϵ�������_____________�����ձ����粻��Ӳֽ�壬����õ��к�����ֵ_______(�ƫ�� ��ƫС������Ӱ�족)�����к��Ȧ�H��________(ȡС�����һλ)��