��Ŀ����
����Ŀ��ij��ѧ��Ӧ�У��跴Ӧ���������ΪE1���������������ΪE2��
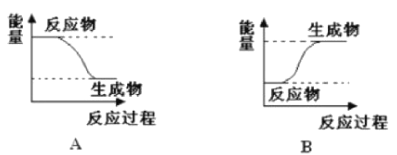
(1)��E1>E2����÷�ӦΪ_____________(��������������������)��Ӧ���÷�Ӧ����ͼ_____________(����A������B��)��ʾ��
(2)��E1��E2����÷�ӦΪ_____________(��������������������)��Ӧ���÷�Ӧ����ͼ___________(����A������B��)��ʾ
(3)̫���ܵĿ�����������21���͵�һ����Ҫ���⡣
�����ô��ܽ��ʴ���̫���ܵ�ԭ���ǰ�����̫�������£�ij�����ۻ����������������������ͷų���Ӧ�������Ӷ�ʹ���µ��Ե��ڡ���֪�������ݣ�
�� | �۵�/�� | �ۻ�����KJ��mol-1 | �ο��۸�/Ԫ |
CaCl2��6H2O | 29.0 | 37.3 | 780850 |
Na2SO4��l0H2O | 32.4 | 77.0 | 800900 |
Na2HPO4��12H2O | 36.1 | 100.1 | 1600-2000 |
Na2S2O3��5H2O | 48.5 | 49.7 | 1400-1800 |
���������������ܽ��ʵ�һ������_____________(����ĸ)��
A CaCl2��6H2O
B Na2SO4��l0H2O
C Na2HPO4��12H2O
D Na2S2O3��5H2O
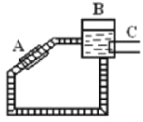
��ͼ��һ��̫������ˮ����ʾ��ͼ��ͼ��A�Ǽ�������B�Ǵ�ˮ������C�ǹ�����ʱ���ȵĸ��������������ݶ�ˮ���ܶȵ���ʶ�������������������ˮ����_________(����˳����������)ʱ�뷽��������
���𰸡����� A ���� B B ˳
��������
��1�����ݷ�Ӧ���������ΪE1���������������ΪE2����Դ�С�жϣ�
��2�����ݷ�Ӧ���������ΪE1���������������ΪE2����Դ�С�жϣ�
��3�����ݱ�����Ϣ�ж��������ʵ��۵���40�����£����ҵ�λ�����������ۻ�ʱ���յ�������ȷ�����з��������ǣ��Ӷ��ó���ȷ�Ľ��ۣ�
��4��������ˮ����ˮ�ܶȲ�ͬ��
��1������Ӧ���������ΪE1�����������������ΪE2����Ӧ�����зų���������Ϊ���ȷ�Ӧ��
�ʴ�Ϊ�����ȣ�A��
��2������Ӧ���������ΪE1С���������������ΪE2����Ӧ������������������Ϊ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�B��
��3��ѡ�������Ӧ�þ��е��ص��ǣ��ڰ�����̫�������£�ij�����ۻ����ۻ�ʱ��λ������������������Ӧ����ࡣͬʱ�۸���̫�ߣ�Na2SO410H2O���Լ۱���ߣ�
�ʴ�ѡB��
��4���������е�ˮ��̫����ɹ�Ⱥ��ܶȱ�С���ܵ����������ع������Ϸ��˶��γ�˳ʱ�뷽��������ˮ�����ʴ�Ϊ��˳��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣���ش��й����⣺
�������� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
(1)���л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ____________��
(2)����Ԫ�����ڱ��е�λ����________________________��
(3)�õ���ʽ��ʾ��Ԫ�����Ԫ���γɻ�����Ĺ���________________________��
(4)����������֤�ܢ���Ԫ�ؽ�����ǿ����ʵ����_______________________��(����ĸ����)
(a)���ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ����ˮ��
(b)����״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
(c)����״����С��ͬ����Ԫ�صĵ��ʷֱ����ˮ���ã��������̪
(d)���Ƚ�������Ԫ�ص���̬�⻯����ȶ���
(5)�͢������ӻ�ԭ�Խ�ǿ����(��������)��____________��һ���û���Ӧ֤ʵ��һ����(д��ѧ����ʽ)____________________________________��



