题目内容
海水是巨大的资源宝库,海水淡化及其综合利用具有重要意义。

完成下列填空:
(1)氯碱工业主要以食盐为原料。为了除去粗盐中的Ca2+、Mg2+、SO42-及泥沙,可将粗盐溶于水,然后进行下列操作,正确的操作顺序是 。
①过滤;②加过量的NaOH溶液;③加适量的盐酸;④加过量的Na2CO3溶液;⑤加过量的BaCl2溶液
a.②⑤④①③ b.①④②⑤③ c d.⑤②④①③
(2)在实验室中可以用萃取的方法提取溴,可选用的试剂是________________,所用主要仪器的名称是____________________。
(3)步骤Ⅰ中用硫酸酸化可提高Cl2利用率的原因是 。
(4)步骤II反应的离子方程式__________________________________________。
(5)海水提溴蒸馏过程中,温度应控制在80~90℃,温度过高或过低都不利于生产 ,请解释原因 。
(6)Mg(OH)2沉淀中混有Ca(OH)2,可选用__________溶液进行洗涤除去。如直接加热Mg(OH)2得到MgO,再电解熔融MgO制金属镁,这样可简化实验步骤,你_______(选填“同意”,“不同意”)该说法,理由是 。
(1)AD
(2)CCl4(或苯);分液漏斗
(3)酸化可抑制Cl2 、Br2与水反应
(4)Br2+SO2+2H2O→4H++SO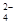 +2Br—(2分)
+2Br—(2分)
(5)温度过高,大量水蒸气随水排除出,溴蒸气中水增加;温度过低,溴不能完全蒸出,吸收率低。(2分)
(6)MgCl2。不同意;MgO熔点很高,熔融时耗能高,增加生产成本。(2分)
【解析】
试题分析:(1)除杂时为了把杂质除净除杂试剂需过量,除去粗盐中的Ca2+、Mg2+、SO42-分别选用Na2CO3溶液、NaOH溶液、 BaCl2溶液,前两中试剂可以用适量的盐酸除去, BaCl2溶液只能放在加入Na2CO3溶液步骤前面,用其除去,注意加盐酸前过滤除去沉淀,故操作顺序为②⑤④①③ 或⑤②④①③;
(2)萃取试剂不能与水互溶且被提取的物质在试剂中的溶解度要远大于在水中的溶解度,实验在分液漏斗中进行;
(3)Cl2 、Br2与水反应是可逆反应,酸化可抑制Cl2 、Br2与水反应;
(5)温度过高,大量水蒸气随水排除出,溴蒸气中水增加;温度过低,溴不能完全蒸出,吸收率低。
(6)选用MgCl2溶液,使Ca(OH)2转化为Mg(OH)2,同时洗去杂质;MgO熔点很高,熔融时耗能高,增加生产成本,故不用电解熔融MgO制金属镁。
考点:考查实验基本操作有关问题。

 怎样学好牛津英语系列答案
怎样学好牛津英语系列答案 导学教程高中新课标系列答案
导学教程高中新课标系列答案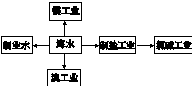
| A、海水制淡水主要有蒸馏法、电渗析法、离子交换法等 | B、海水制盐、发展氯碱工业都是发生物理变化 | C、海水提溴过程中先通入Cl2将溴离子氧化为溴单质 | D、工业上用电解熔融MgCl2的方法制取金属镁 |





