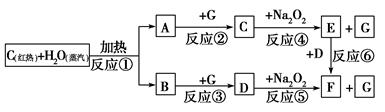
��Ŀ����
����̼������ͭ�ȳ���Ԫ���ڻ������г����ֳ����ּ�̬��������-2��-1��+2��+4��+6�ȼ�̬����ЩԪ���ڻ���������������ҪӦ�á�
��1��31.2gþ��̼�۵Ļ������һ��������ǡ����ȫ��Ӧ���ټ�������ˮ���õ�40.6g��ɫ������ͬʱ�����ܶ�Ϊ1.4107g/L����״�����ı���ϩ�Ͳ�������X�Ļ�����塣
��þ��̼�۵ķ�Ӧ����Ļ�ѧʽΪ ______________ ��
��ԭ�������̼�۵����ʵ�������Ϊ ___________������С����ʾ������2λС����
��2��ij������������Ԫ����ɣ������������Ӻ����������ӡ�ȡ21.76g�ø����Σ�ƽ����Ϊ���ݡ�����һ���������������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ�����ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ���������˺�����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣
�ٸø����εĻ�ѧʽΪ_____________��
�ڸø����������������ӵ�������Ϊ _________________ ��
��3��ij����ѧ�Ҿ����ⷢ��һ���µĴŻ�������Ҫ�ɷ�ΪFe1-xS1+x�����ʲ���Fe��S��������֪�ôŻ������У���Ԫ��������������������Ϊ75%��ȡ5�� 73%�ĸôŻ������ýӴ��������ᣬ������¯�����յ������Ϊ4%���Ӵ����з�Ӧ��ת����Ϊ94%��SO3������Ч��Ϊ97%���������Ƶ�98.3%��Ũ�����������________________��������2λС����
��1����MgC2��Mg2C3 ��0.63
��2����Cu5S(SO4)2 ��+1�۵�ͭ��+2�۵�ͭ=4:1
��3��6.08t
�������������
��1���ٸ��������֪��̼���V��Ӧ�õ��Ļ�������ˮ��Ӧ�IJ������ϩC3H4������ݻ��������������ϼ۵Ĵ�����Ϊ0��ȷ�������к���Mg2C3������ϩC3H4����Է�������Ϊ40������ϩ�Ͳ�������X�Ļ�������״�����ܶ�Ϊ1.4107g/L����ƽ����Է�������Ϊ1.4107��22.4=31.6.˵��������������Է�������С��31.6��ֻ��C2H2��C2H4�����ݻ��������������ϼ۵Ĵ�����Ϊ0��������C2H2��ȷ�������л����к���MgC2��������C2H4��ȷ�������л����к���MgC�����ʵ����ȥ�����þ��̼�۵ķ�Ӧ����Mg2C3��MgC2���ٹ������õ���Mg(OH)2�����ʵ���Ϊn(Mg(OH)2)="m��M=" 40.6g�� 58g/mol =0.7mol.����ԭ31.2gþ��̼�۵Ļ���ﺬ�е�C�����ʵ���Ϊn(C)=(31.2g��0.7mol�� 24g/mol) ��12 g/mol=1.2mol�� ԭ�������̼�۵����ʵ�������Ϊ1.2mol��(0.7+1.2)mol=0.63.
��2������һ�����������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ��֤������SO42-�������ʵ���Ϊn(SO42-)="9.32g��233g/mol=0.04mol;" ���ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ����������13.98g>9.32g֤��ԭ�����л�����S2-������������������������SO42-����n(SO42-) (��)="13.98g��233g/mol=0.06mol;" n(S2-)="0.06-0.04=0.02mol." ����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣֤������CuԪ��n(Cu2+)=" 8.00g��80g/mol=0.1mol." n(Cu2+): n(S2-): n(SO42-)=0.1:0.02:0.04=5:1:2.���Ը��εĻ�ѧʽΪCu5S(SO4)2 �ڸ�����Cu�Ļ��ϼ���+1��+2���ּ�̬������+1�۵�Ϊx����+2�۵�Ϊ(5-x).���ݻ��������������ϼ۵Ĵ�����Ϊ0�ɵ�x+2(5-x)=2+4.���x=4����5-x=1.����m(Cu+):m(Cu2+)=4:1.
��3���ôŻ������У���Ԫ��������������������Ϊ75%�������������������Ϊ25%��n(Fe3+):n(Fe2+)=3:1.���ݻ��������������ϼ۵Ĵ�����Ϊ0����ȷ����������S��ԭ�Ӹ���Ϊ3��3��1��2=11.���Ըû�����Ļ�ѧʽΪFe4S11.���������֪���ת��Ϊ�����S�����ʵ���Ϊ{(5��106g��73%��(1��4%)��94%��97%)��576g/mol}��11=6.1��104mol.���Եõ���98.3%��Ũ����������ǣ�6.1��104mol��98g/mol����98.3%=6.08��106g="6.08t" ��
���㣺�������ʻ�ѧʽ��ȷ������ɳɷֵĺ�����ԭ�ϵ������ʡ���Ʒ�IJ��ʵļ����֪ʶ��

����ͬ״���µ�12C18O��14N2�������壬����˵����ȷ���� �� ��
| A����������ȣ������������ | B����ԭ������ȣ������������ |
| C������������ȣ��������� | D���������ȣ����ܶ���� |
��84����Һ������Чɱ�����H1N1������ijͬѧ������һƿijƷ�ơ�84����Һ����
������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25% NaClO��1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á������������Ϣ�����֪ʶ�ش��������⣺
(1)�á�84����Һ�������ʵ���Ũ��Ϊ________mol��L��1��
(2)��ͬѧȡ100 mL��Ʒ�ơ�84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na�� )��________mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)��ͬѧ���ĸ�Ʒ�ơ�84����Һ�����䷽������NaClO��������480 mL��25% NaClO������Һ������˵����ȷ����________��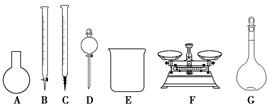
| A����ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ������� |
| B������ƿ������ˮϴ����Ӧ��ɲ���������Һ���� |
| C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ�� |
| D����Ҫ������NaClO��������Ϊ143 g |
��֪���ᡢ��ˮ���ܶ�������ˮ�����Ĺ�ϵ��ͼ��ʾ�����������백ˮ��һ�ݣ�����ݱ�����Ϣ���ش��������⣺
| | ���ʵ����ʵ��� Ũ��/mol��L��1 | ��Һ���ܶ�/g��cm��3 |
| ���� | c1 | ��1 |
| ��ˮ | c2 | ��2 |
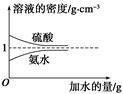
��1�������������������Ϊ________(��д��λ���ú�c1����1�Ĵ���ʽ��ʾ)��
��2�����ʵ���Ũ��Ϊc1 mol��L��1��������ˮ��������(��Ϻ���Һ������仯���Բ���)��������Һ�����ʵ���Ũ��Ϊ________ mol��L��1��
��3�������ʵ���Ũ�ȷֱ�Ϊc2 mol��L��1��
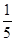 c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________
c2 mol��L��1�İ�ˮ��������ϣ�������Һ���ܶ�________(����ڡ�����С�ڡ����ڡ�����ͬ)��2 g��cm��3��������Һ�����ʵ���Ũ��________ 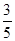 c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��
c2 mol��L��1(���Ϻ���Һ������仯���Բ���)��  2C(g)������2�����C��Ũ��Ϊ0.6mol/L��
2C(g)������2�����C��Ũ��Ϊ0.6mol/L��