��Ŀ����
��14�֣���ͼ��һ����ȡ������������Ϊԭ�Ͻ���ij���ض���Ӧ�о���װ�á�
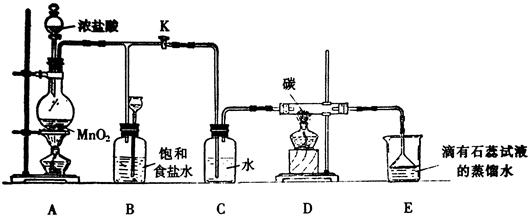
��1��ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ���K��ʹCl2��������װ�ã��ٵ�ȼD���ƾ��ƣ�Ȼ��������Eװ�ã�E��ʯ����Һ�ȱ��Ȼ��Ϊ��ɫ��ͬʱ©���е�Һ�������������������ɫ�仯��ԭ����
a����Ӧ�в���CO2��Ե�� b����Ӧ�в���HCl��Ե��
c����Ӧ�в���HCl����Cl2����ˮ d����Ӧ��ͬʱ��CO2��HCl������Ե��
D����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��C�������� ��
��3������E���е�Һ���Ϊ����ʯ��ˮ����Ӧ�����е�����Ϊ ��
a���а�ɫ�������� b�������ɰ�ɫ�������������ʧ
c������������ d����ʼ������Ȼ�������ɫ����
��4������Ӧ������ر�K����ȥA���ƾ��ƣ������������ã�A������Cl2��������ʱB������Ϊ ��B�������� ��
��
��5��Eװ����ȷ��D����Ӧ����CO2������Ϊ��֤��CO2�Ĵ��ڣ�Ҫ��Eװ�ý��иı䣬����װ�÷���Ҫ����� ��
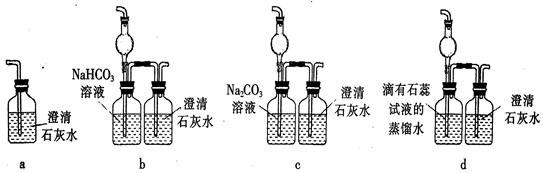
��6����ʵ���Ŀ���� ��

��1��ʵ�鿪ʼʱ���ȵ�ȼA���ľƾ��ƣ���K��ʹCl2��������װ�ã��ٵ�ȼD���ƾ��ƣ�Ȼ��������Eװ�ã�E��ʯ����Һ�ȱ��Ȼ��Ϊ��ɫ��ͬʱ©���е�Һ�������������������ɫ�仯��ԭ����
a����Ӧ�в���CO2��Ե�� b����Ӧ�в���HCl��Ե��
c����Ӧ�в���HCl����Cl2����ˮ d����Ӧ��ͬʱ��CO2��HCl������Ե��
D����Ӧ�Ļ�ѧ����ʽΪ ��
��2��װ��C�������� ��
��3������E���е�Һ���Ϊ����ʯ��ˮ����Ӧ�����е�����Ϊ ��
a���а�ɫ�������� b�������ɰ�ɫ�������������ʧ
c������������ d����ʼ������Ȼ�������ɫ����
��4������Ӧ������ر�K����ȥA���ƾ��ƣ������������ã�A������Cl2��������ʱB������Ϊ ��B��������
 ��
����5��Eװ����ȷ��D����Ӧ����CO2������Ϊ��֤��CO2�Ĵ��ڣ�Ҫ��Eװ�ý��иı䣬����װ�÷���Ҫ����� ��

��6����ʵ���Ŀ���� ��
��1��c ��2�֣� 2Cl2+C+2H2O
 CO2+ 4HCl ��2�֣�
CO2+ 4HCl ��2�֣���2���ṩˮ������2�֣� ��3��c��2�֣�
��4��B��Һ�屻ѹ�볤��©���У�1�֣������������������ֹ��Ⱦ��1�֣�
��5��d��2�֣�
��6��̽����ʪ��������̼�ڼ��������µķ�Ӧ��̽��Cl2��C��H2O�ض���Ӧ�������ʵ�飩��2����������Ҳ���֣�
��

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
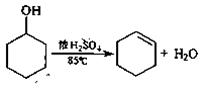
 +4HCl��Ũ��
+4HCl��Ũ�� MnCl
MnCl