��Ŀ����
�Ҵ���ͨ�����۵�������ԭ�Ϸ����Ƶã����ڿ�������Դ��ͨ���Ҵ���ȡ�����������õ�Ӧ��ǰ������֪ͨ���Ҵ���ȡ��������������·�ߣ�
a.ˮ������������CH3CH2OH(g) ��H2O(g)®4H2(g) ��2CO(g) DH =��255.58 kJ��mol-1
b.���ִ�������CH3CH2OH(g)��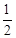 O2(g)®3H2(g)��2CO(g)
DH =��13.76kJ��mol-1
O2(g)®3H2(g)��2CO(g)
DH =��13.76kJ��mol-1
������˵���������( )
A.��ԭ�����ĵĽǶ�������a·��������м�ֵ
B.���������ĵĽǶ�����,b·�������������
C.a·����������Ҫ���ĺܶ�������������ʵ�����������岻��
D.��b·�߽���,��ϵ�������ή��
���𰸡�
D
���������Ƚ�����·�ߣ�������ͬ��������b���ĵ��Ҵ��϶࣬��a·�ߺ��ܴ���ʵ�����������岻������Ӧ��Ϊ���ȷ�Ӧ����ϵ�����������ߣ�����D��������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��֪ͨ���Ҵ���ȡ��������������·�ߣ�
a��CH3CH2OH��g��+H2O��g���T4H2��g��+2CO��g����H=+255.6kJ?mol-1
b��CH3CH2OH��g��+
O2��g���T3H2��g��+2CO��g����H=+13.8kJ?mol-1
������˵������ȷ���ǣ�������
a��CH3CH2OH��g��+H2O��g���T4H2��g��+2CO��g����H=+255.6kJ?mol-1
b��CH3CH2OH��g��+
| 1 |
| 2 |
������˵������ȷ���ǣ�������
| A�������¶ȣ������b·�����Ҵ���ת���� |
| B�����������ĵĽǶ�������b·������������� |
| C���Ҵ���ͨ�����۵�����ԭ�Ϸ����Ƶã����ڿ�������Դ |
| D����a��b֪��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ?mol-1 |
��֪ͨ���Ҵ���ȡ��������������·�ߣ�
a��CH3CH2OH(g)+H2O(g)==4H2(g)+2CO(g) ��H= +255.6kJ��mol��1
b��CH3CH2OH(g)+1/2O2(g)==3H2(g)+2CO(g) ��H= +13.8kJ��mol��1
������˵������ȷ����
| A�������¶ȣ������b·�����Ҵ���ת���� |
| B�����������ĵĽǶ�������b·������������� |
| C���Ҵ���ͨ�����۵�����ԭ�Ϸ����Ƶã����ڿ�������Դ |
| D����a��b֪��2H2(g)+O2(g)��2H2O(g)��H=��483.6kJ��mol��1 |