��Ŀ����
�о����������������ڴ����к������ӵ������ʱ���漰���·�Ӧ��
2NO2(g)+NaCl(s)?NaNO3(s)+ClNO(g) K1 ��H1��0(I)
2NO(g)+Cl2(g)?2ClNO(g) K2 ��H2��0(II)
(1)4NO2(g)+2NaCl(s)?2NaNO3(s)+2NO(g)+Cl2(g) K3 ��H3
K3 = (��K1��K2��ʾ)(K1��K2��K3��Ϊƽ�ⳣ��)
(2)Ϊ�о���ͬ�����Է�Ӧ(II)��Ӱ�죬�ں��������£���2L�����ܱ������м��� 0.2mol��NO��0.1mol�� Cl2��10minʱ��Ӧ(II)�ﵽƽ�⡣���10min�� V(ClNO)= 7.5��10��3mol/(L•min)����ƽ��� n(Cl2)= mol��NO��ת����a1= �� �����������䣬��Ӧ(II)�ں�ѹ�����½��У�ƽ��ʱNO��ת����a2 a1(���������������=��)�� ƽ�ⳣ��K2 (���������С�����䡱)����ҪʹK2��С���ɲ�ȡ�Ĵ�ʩ��__________________��
(3)ʵ���ҿ���NaOH��Һ����NO2����ӦΪ 2NO2 + 2 NaOH = NaNO3 + NaNO2 + H2O����0.2mol NaOH��ˮ��Һ�� 0.2mol NO2ǡ����ȫ��Ӧ�� 1L��ҺA����ҺB Ϊ 0.1mol/L��CH3COONa��Һ��������Һ�� c(NO3��)��c(NO2��)����c(CH3COO��)�ɴ�С��˳��Ϊ______________________(��֪ HNO2�ĵ��볣��Ϊ Ka= 7.1��10��4mol/L��CH3COOH�ĵ��볣��Ϊ Ka= 1.7��10��5 mol/L )��ʹ��ҺA����ҺB��PH��ȵķ�����
Ka= 1.7��10��5 mol/L )��ʹ��ҺA����ҺB��PH��ȵķ�����
A������ҺA�м�����ˮ B������ҺA�м�����Na OH
OH
C������ҺB�м�����ˮ D������ҺB�м�����NaOH


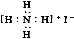
 NH3(g)��H2S(g)��ijһ�¶��´ﵽƽ�⣬���и�������У���ʹ��ѧƽ�������ƶ�����( )
NH3(g)��H2S(g)��ijһ�¶��´ﵽƽ�⣬���и�������У���ʹ��ѧƽ�������ƶ�����( ) 2NH3 ( g ) ��H = -92.0 kJ/mol�������¶��µ�1 mol N2 ��3 mol H2 ����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(����������ʧ)
2NH3 ( g ) ��H = -92.0 kJ/mol�������¶��µ�1 mol N2 ��3 mol H2 ����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų�������Ϊ(����������ʧ)
 Cr2O72-(aq)+H2O����25��ʱ��ȡNa2CrO4��Һ�����ữʵ�飬��ò���ʵ����������:
Cr2O72-(aq)+H2O����25��ʱ��ȡNa2CrO4��Һ�����ữʵ�飬��ò���ʵ����������:
