��Ŀ����
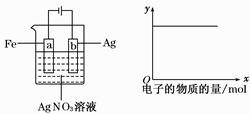
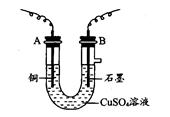
����ͼ��ʾ��װ���У���ͨ��ֱ����5 minʱ��ͭ�缫��������2.16 g���Իش�
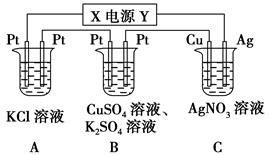
��1����Դ�缫X������Ϊ________��
��2��pH�仯��A________��B________��C________��(�������С�����䡱)
��3��ͨ��5 min��B�й��ռ�224 mL����(��״��)����Һ���Ϊ200 mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
��4����A��KCl��Һ�����Ҳ��200 mL��������Һ��OH�������ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��

��1����Դ�缫X������Ϊ________��
��2��pH�仯��A________��B________��C________��(�������С�����䡱)
��3��ͨ��5 min��B�й��ռ�224 mL����(��״��)����Һ���Ϊ200 mL����ͨ��ǰCuSO4��Һ�����ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
��4����A��KCl��Һ�����Ҳ��200 mL��������Һ��OH�������ʵ���Ũ��Ϊ________(����ǰ����Һ����ޱ仯)��
��1����������2������С�����䡡��3��0.025 mol��L��1 ��4��0.1 mol��L��1
�����������1��Cװ�õ�ͭ�缫�������ӣ�˵��ͭ�����н�������������Һ�е������ӱ���ԭ���������ʣ���ͭ��Ϊ�������ɴ˿�ȷ��X��Ϊ��������2��Aװ���ǵ��KCl��Һ��������������������������������Һ������������Ũ������Bװ����������������ͭ����ͭ�����������������������������������������Һ��������Ũ������Cװ�����������������ʣ������ϵ���ʧȥ���ӱ�������ӣ�������AgNO3��Һ�����ʵ���Ũ�Ȳ��䡣��3��Bװ�������ϵ���ת�Ƶ���Ŀ��Cװ����ת�Ƶĵ�����Ŀ��ͬ��Cװ����ת�Ƶĵ���Ϊ
 ��0.02 mol�����жϣ�Bװ����������Cu2����2e��=Cu,2H����2e��=H2����������4OH����4e��=O2����2H2O����������ɵã�2n(H2)��2n(Cu)��4n(O2)��0.02 mol��n(H2)��n(O2)��
��0.02 mol�����жϣ�Bװ����������Cu2����2e��=Cu,2H����2e��=H2����������4OH����4e��=O2����2H2O����������ɵã�2n(H2)��2n(Cu)��4n(O2)��0.02 mol��n(H2)��n(O2)��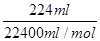 �����n(Cu)��0.005 mol��CuSO4��Һ���ʵ���Ũ��Ϊ��
�����n(Cu)��0.005 mol��CuSO4��Һ���ʵ���Ũ��Ϊ��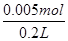 ��0.025 mol��L��1����4��Aװ�õķ�ӦΪ��2KCl��2H2O
��0.025 mol��L��1����4��Aװ�õķ�ӦΪ��2KCl��2H2O 2KOH��H2����Cl2��������Ӧ�е���ת�Ƶ����ʵ��������ɵ����������ӵ����ʵ�����ȣ�Ϊ0.02 mol��c(OH��)��
2KOH��H2����Cl2��������Ӧ�е���ת�Ƶ����ʵ��������ɵ����������ӵ����ʵ�����ȣ�Ϊ0.02 mol��c(OH��)��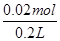 ��0.1 mol��L��1
��0.1 mol��L��1
��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ
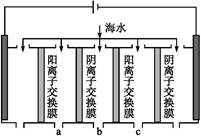
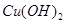 ��ǡ�ûָ������ǰ��pH��Ũ�ȣ����������ת�Ƶĵ��ӵ����ʵ���Ϊ��
��ǡ�ûָ������ǰ��pH��Ũ�ȣ����������ת�Ƶĵ��ӵ����ʵ���Ϊ��