��Ŀ����
����Ŀ���ڢ�A����A��Ԫ�ؼ��仯����������������й㷺Ӧ�á��ش��������⣺
(1)��̬��ԭ�Ӻ�������Ų�ʽΪ___________��
(2)H2�����й���ص���ʽ��___________ ( ����)��
A��s-s B��s-p C��p-p
(3)OF2��O2F2�Ƿ����OF2���ӵ�����ԭ���ӻ�������___________��
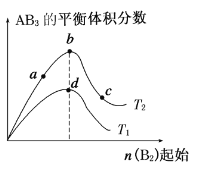
(4)�ơ��ؾ�����ͼA��ʾ����ѻ���ʽ��___________��

(5)�Ȼ��ƾ�����ͼB��ʾ(�������Cl-���������Na+)�����������ӵ���λ��Ϊ___________��
���𰸡�1s22s22p43s1Asp3���������ѻ�6
��������
(1)Na��11��Ԫ�أ����ڵ�������IA�壬��������Ų�ʽΪ��1s22s22p43s1���ʴ�Ϊ��1s22s22p43s1��
(2)H2������1s�����1s����ص����ʴ�Ϊ��A��
(3)OF2���ӵ�����Oԭ�ӹµ��Ӷ���=![]() =2���ӻ������Ŀ=2+2=4��Oԭ���ӻ���ʽΪsp3���ʴ�Ϊ��sp3��
=2���ӻ������Ŀ=2+2=4��Oԭ���ӻ���ʽΪsp3���ʴ�Ϊ��sp3��
(5)�ɾ����ṹͼ����֪�ơ��ؾ���Ϊ���������ѻ����ʴ�Ϊ�����������ѻ���
(6)������Na+����λ��Ϊ6��������Cl-��Ŀ=8��![]() +6��
+6��![]() =4��Na+������Ŀ=1+12��
=4��Na+������Ŀ=1+12��![]() =4��������Ŀ֮��Ϊ1��1���ʶ�����λ����ͬ����Cl-���ӵ���λ��Ϊ6���ʴ�Ϊ��6��
=4��������Ŀ֮��Ϊ1��1���ʶ�����λ����ͬ����Cl-���ӵ���λ��Ϊ6���ʴ�Ϊ��6��

����Ŀ��TKʱ����2.0L�����ܱ������г���0.10molCOCl2,������ӦCOCl2(g)![]() Cl2(g)+CO(g)����һ��ʱ���Ӧ�ﵽƽ�⡣��Ӧ�����в�õIJ������ݼ��±�������˵����ȷ����:
Cl2(g)+CO(g)����һ��ʱ���Ӧ�ﵽƽ�⡣��Ӧ�����в�õIJ������ݼ��±�������˵����ȷ����:
t/s | 0 | 2 | 4 | 6 | 8 |
n(Cl2)/mol | 0 | 0.030 | 0.039 | 0.040 | 0.040 |
����˵����ȷ����
A. TKʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ1/75
B. ��Ӧ��ǰ2s��ƽ���ٶ�v(CO)=0.015mol��L-1��s-
C. ���������������䣬�����¶ȣ�ƽ��ʱc(Cl2)=0.022molL-1����Ӧ�ġ�H��0
D. ƽ������ϻ��������ٳ���0.10molCOCl2,ƽ�������ƶ���COCl2��ת��������