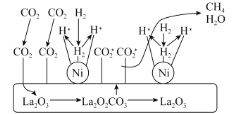
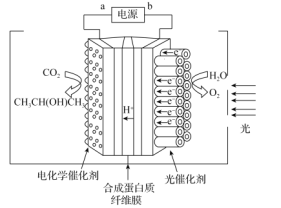
��Ŀ����
����Ŀ���п�Ժ���ݻ�ѧ�����о�����Fe3(CO)12/ZSM��5��CO2����ϳɵ�̼ϩ����Ӧ�����ò��ﺬCH4��C3H6��C4H8�ȸ������Ӧ������ͼ
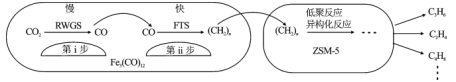
����˵����ȷ����
A.��i����ӦΪCO2��H2��CO��H2O
B.��i����Ӧ�Ļ�ܵ��ڵ�ii��
C.Fe3(CO)12/ZSM��5ʹCO2����ϳɵ�̼ϩ����H��С
D.���Ӳ�ͬ������Ӧ��ƽ�ⳣ��������ͬ
���𰸡�A
��������
A����i������ӦΪ��CO2+l2��CO+H2O ��A��ȷ��
B���ڢ�����Ӧ������Ӧ���ڢ����ǿ췴Ӧ����Ӧ�Ļ��Խ�ͣ���Ӧ����Խ�죬���Ե�i�� ��Ӧ�Ļ�ܸ��ڵڢ�������B����
C���������ܸı�ƽ��״̬�����ܸı��ʱ��H����C����
D����Ӧ��ƽ�ⳣ��ֻ���¶��йأ���ͬ�����ܸı䷴Ӧ���̣������ܸı䷴Ӧ����ʼ״̬�����ܸı䷴Ӧ�¶ȣ����Է�Ӧ��ƽ�ⳣ�����䣬��D����
�ʴ�Ϊ��A��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���Ƶij������ϼ�Ϊ��3�ۣ��ҹ��̲��ŷḻ�ĺ��ƿ�ʯ(Y2FeBe2Si2O10)����ҵ��ͨ�������������̿ɻ�������ơ�
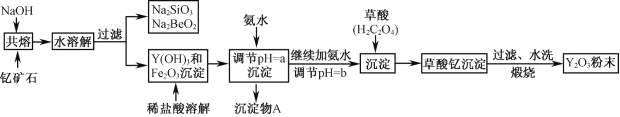
��֪���ٸ��������йؽ��������γ������������ʱ��pH���±���
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe3�� | 2.1 | 3.1 |
Y3�� | 6.0 | 8.2 |
����Ԫ�����ڱ��У���Ԫ�غ���Ԫ�ش��ڵڶ����ں͵������ڵĶԽ���λ�ã���ѧ�������ơ�
(1)д��Na2SiO3��һ����;________________________��
(2)����Na2SiO3��Na2BeO2�����Һ���Ƶ�Be(OH)2������
�� ���ѡ�������_______�����Լ�����ͨ����Ҫ�IJ�������ʵ�֡�
A��NaOH��Һ B����ˮ C��CO2 D��HNO3
�� д��Na2BeO2���������ᷢ����Ӧ�����ӷ���ʽ___________________________��
(3)�����£���ӦFe3����3H2O(g) ![]() Fe (OH)3����3H+��ƽ�ⳣ��K= ______��ΪʹFe3��������ȫ���ð�ˮ����pH��aʱ��aӦ������_________��Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ____________________________��
Fe (OH)3����3H+��ƽ�ⳣ��K= ______��ΪʹFe3��������ȫ���ð�ˮ����pH��aʱ��aӦ������_________��Χ�ڣ������Ӱ�ˮ����pH =b������Ӧ�����ӷ���ʽΪ____________________________��
(4)���ղ�����ʱ�����ֽⷴӦ����������Ϊ�����ƣ����������ʹ����ʯ��ˮ����ǡ�д��������[Y2(C2O4)3��nH2O]���յĻ�ѧ����ʽ___________________________________��