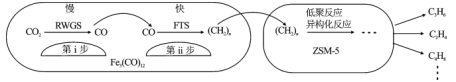
��Ŀ����
����Ŀ��̼����Դ�Ĵ�������ʹ������CO2Ũ�ȳ������ϵ����ӣ���CO2Ϊԭ�ϼ���ϳɣ��������Դ���ʾ��нϺõķ�չǰ�����ش��������⣺
(1)CO2��(�������)����ϳɼ�����̷�������������Ӧ��
����Ӧ��CO2(g)+4H2(g)![]() CH4(g)+2H2O(g) ��H1=akJ��mol-1
CH4(g)+2H2O(g) ��H1=akJ��mol-1
����Ӧ��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2=41.1kJ��mol-1
CO(g)+H2O(g) ��H2=41.1kJ��mol-1
��֪��صĻ�ѧ�������������£�

��a=___������ϳɼ���ʱ��ͨ�������¶�Ϊ500�棬���ܹ���Ҳ���˹��͵�ԭ����___��
(2)Ϊ�����CO2������CH4������CH4ѡ����(CH4ѡ����=![]() ��100%)����Ҫ��ͨ���Դ����ĺ���ѡ����ʵ�֡�
��100%)����Ҫ��ͨ���Դ����ĺ���ѡ����ʵ�֡�
��CO2������CH4��һ�ִ�������ͼ������˵����ȷ����___(����)��
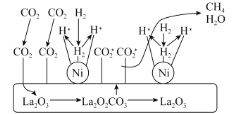
A.������ʹ�õĴ���ΪLa2O3��La2O2CO3
B.La2O2CO3�����ͷų�CO2*(�����)
C.H2����Ni���������ѽ�����̬H*�Ĺ���Ϊ���ȹ���
D.CO2������CH4�Ĺ�����ҪLa2O3��Ni��ͬ�����
�ڱ���500�治�䣬��1L�ܱ������г���4molCO2��12molH2������Ӧ������ʼѹǿΪp��20min����������Ӧ���ﵽƽ��״̬����ô�ʱc(H2O)=5mol��L-1����ϵѹǿ��Ϊ0.75p������������Ӧ���ۺ���ЧӦΪ___��v(CH4)=___mol��L-1��min-1��CH4ѡ����=___(������λ��Ч����)������Ӧ��ƽ�ⳣ��K=___��
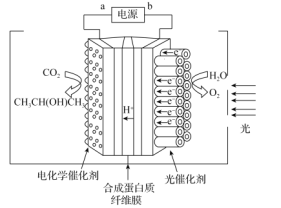
(3)CO2�Ĺ�����Ӧ����ͼ��ʾ����TiO2Ϊ������ͨ�����ˮ�������Ӻ����ӣ����ݵ�����(Pt/CNT)�յ���������ԭCO2�Ƶ��������
����������������ȸ���Ӧ������Ϊ��ѡ���˵绯ѧ��������������___��
����������������ĵ缫��ӦΪ___��
���𰸡�-156.9 �¶ȵ���500������Ӧ���ʵͣ��¶ȸ���500����ƽ��������CO�ķ����ƶ��̶����������ڼ�������� BD �ų�272.7kJ 0.1 66.7% ![]() (��0.62) �������нϸߵĴ����ԺͲ���ѡ���ԣ����������ⷴӦ 3CO2+18H++18e-=CH3CH(OH)CH3+5H2O
(��0.62) �������нϸߵĴ����ԺͲ���ѡ���ԣ����������ⷴӦ 3CO2+18H++18e-=CH3CH(OH)CH3+5H2O
��������
(1)������H=��Ӧ����ܼ���-��������ܼ��ܼ���aֵ���¶ȼ�Ӱ�췴Ӧ���еĿ�������Ӱ��ƽ���ƶ��ķ���
(2)��A������CO2������CH4�Ĵ�����ͼʾ��������ʹ�õĴ���ΪLa2O3��La2O2CO3Ϊ�м���
B������ͼʾ��La2O2CO3�����ͷų�CO2*(�����)��
C��H2����Ni���������ѽ�����̬H*�Ĺ���Ϊ�Ͽ���ѧ���Ĺ��̣�
D������ͼʾ��La2O3�ǽ�CO2ת��ΪCO2*(�����)�Ĵ�����Ni�ǽ�H2ת��Ϊ�̬H*�Ĵ�����
����ƽ��ʱ����CH4�����ʵ���Ϊxmol����������ӦCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)������2xmol H2O(g)������xmol CO2��4xmol H2����ƽ��ʱ����CO�����ʵ���Ϊymol�����ݸ���ӦCO2(g)+H2(g)
CH4(g)+2H2O(g)������2xmol H2O(g)������xmol CO2��4xmol H2����ƽ��ʱ����CO�����ʵ���Ϊymol�����ݸ���ӦCO2(g)+H2(g)![]() CO(g)+H2O(g)������ymol H2O(g)������ymol CO2��ymol H2����ƽ��ʱʣ��CO2�����ʵ���=(4-x-y)mol��ʣ��H2�����ʵ���=(12-4x-y)mol������ˮ���������ʵ���Ϊ(2x+y)mol��������ƽ��ʱ���c(H2O)=5mol��L-1����n(H2O)=5mol����2x+y=5����ͬ�����£�ѹǿ֮�ȵ������ʵ���֮�ȣ���
CO(g)+H2O(g)������ymol H2O(g)������ymol CO2��ymol H2����ƽ��ʱʣ��CO2�����ʵ���=(4-x-y)mol��ʣ��H2�����ʵ���=(12-4x-y)mol������ˮ���������ʵ���Ϊ(2x+y)mol��������ƽ��ʱ���c(H2O)=5mol��L-1����n(H2O)=5mol����2x+y=5����ͬ�����£�ѹǿ֮�ȵ������ʵ���֮�ȣ���![]() �����x��y���ٸ�������ֱ������
�����x��y���ٸ�������ֱ������
(3)�ٴ����Բ�������ɾ���ѡ���ԣ��ɷ�ֹ����Ӧ�ķ�����
�ڸ������⣬���ͼʾ���Դa��������Ϊ���ص������������õ��ӣ�������ԭ��Ӧ��CO2��CԪ�صĻ��ϼ�Ϊ+4�ۣ������(CH3CH(OH)CH3)��CԪ�صĻ��ϼ�Ϊ-2�ۣ��������������ƶ����ݴ˷�����д�缫��Ӧʽ��
(1)��֪����Ӧ��CO2(g)+H2(g)![]() CO(g)+H2O(g)����H2=41.1kJ��mol-1=��Ӧ����ܼ���-��������ܼ���=E(CO2)+436kJ��mol-1-1076kJ��mol-1-2��463kJ��mol-1���������̼�ܼ���E(CO2)= 41.1kJ��mol-1-436kJ��mol-1+1076kJ��mol-1+2��463kJ��mol-1=1607.1 kJ��mol-1������ӦCO2(g)+4H2(g)
CO(g)+H2O(g)����H2=41.1kJ��mol-1=��Ӧ����ܼ���-��������ܼ���=E(CO2)+436kJ��mol-1-1076kJ��mol-1-2��463kJ��mol-1���������̼�ܼ���E(CO2)= 41.1kJ��mol-1-436kJ��mol-1+1076kJ��mol-1+2��463kJ��mol-1=1607.1 kJ��mol-1������ӦCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)����H1=akJ��mol-1=��Ӧ����ܼ���-��������ܼ���=436 kJ��mol-1��4+1607.1 kJ��mol-1-4��414 kJ��mol-1-4��463 kJ��mol-1=-156.9 kJ��mol-1����a=-156.9���¶ȼ�Ӱ�췴Ӧ���еĿ�������Ӱ��ƽ���ƶ��ķ�����ϳɼ���ʱ���¶ȵ���500������Ӧ���ʵͣ��¶ȸ���500�������ڸ���ӦCO2(g)+H2(g)
CH4(g)+2H2O(g)����H1=akJ��mol-1=��Ӧ����ܼ���-��������ܼ���=436 kJ��mol-1��4+1607.1 kJ��mol-1-4��414 kJ��mol-1-4��463 kJ��mol-1=-156.9 kJ��mol-1����a=-156.9���¶ȼ�Ӱ�췴Ӧ���еĿ�������Ӱ��ƽ���ƶ��ķ�����ϳɼ���ʱ���¶ȵ���500������Ӧ���ʵͣ��¶ȸ���500�������ڸ���ӦCO2(g)+H2(g)![]() CO(g)+H2O(g)������Ӧ���ȣ�ƽ��������CO�ķ����ƶ��̶����������ڼ�������ɣ�
CO(g)+H2O(g)������Ӧ���ȣ�ƽ��������CO�ķ����ƶ��̶����������ڼ�������ɣ�
(2)��A������CO2������CH4�Ĵ�����ͼʾ��������ʹ�õĴ���ΪLa2O3��La2O2CO3Ϊ�м�����A����
B������ͼʾ��La2O2CO3�����ͷų�CO2*(�����)����B��ȷ��
D������ͼʾ��La2O3�ǽ�CO2ת��ΪCO2*(�����)�Ĵ�����Ni�ǽ�H2ת��Ϊ�̬H*�Ĵ������������CH4�Ĺ�����ҪLa2O3��Ni��ͬ����ɣ���D��ȷ��
��ѡBD��
�ڱ���500�治�䣬��1L�ܱ������г���4molCO2��12molH2������Ӧ������ʼѹǿΪp��20min��Ӧ���ﵽƽ��״̬����ϵѹǿ��Ϊ0.75p����ƽ��ʱ����CH4�����ʵ���Ϊxmol����������ӦCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)������2xmol H2O(g)������xmol CO2��4xmol H2����ƽ��ʱ����CO�����ʵ���Ϊymol�����ݸ���ӦCO2(g)+H2(g)
CH4(g)+2H2O(g)������2xmol H2O(g)������xmol CO2��4xmol H2����ƽ��ʱ����CO�����ʵ���Ϊymol�����ݸ���ӦCO2(g)+H2(g)![]() CO(g)+H2O(g)������ymol H2O(g)������ymol CO2��ymol H2����ƽ��ʱʣ��CO2�����ʵ���=(4-x-y)mol��ʣ��H2�����ʵ���=(12-4x-y)mol������ˮ���������ʵ���Ϊ(2x+y)mol��������ƽ��ʱ���c(H2O)=5mol��L-1����n(H2O)=5mol����2x+y=5����ͬ�����£�ѹǿ֮�ȵ������ʵ���֮�ȣ���
CO(g)+H2O(g)������ymol H2O(g)������ymol CO2��ymol H2����ƽ��ʱʣ��CO2�����ʵ���=(4-x-y)mol��ʣ��H2�����ʵ���=(12-4x-y)mol������ˮ���������ʵ���Ϊ(2x+y)mol��������ƽ��ʱ���c(H2O)=5mol��L-1����n(H2O)=5mol����2x+y=5����ͬ�����£�ѹǿ֮�ȵ������ʵ���֮�ȣ���![]() ��
��![]() �����x=2������2x+y=5�ɵ�y=1����������ӦCO2(g)+4H2(g)
�����x=2������2x+y=5�ɵ�y=1����������ӦCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)��H1=-156.9 kJ��mol-1�ɵã�����1mol CH4�ų�������Ϊ156.9kJ����������������ﵽƽ��ʱ����2mol CH4����ų�������=156.9kJ��2=313.8kJ�����ݸ���Ӧ��CO2(g)+H2(g)
CH4(g)+2H2O(g)��H1=-156.9 kJ��mol-1�ɵã�����1mol CH4�ų�������Ϊ156.9kJ����������������ﵽƽ��ʱ����2mol CH4����ų�������=156.9kJ��2=313.8kJ�����ݸ���Ӧ��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2=41.1kJ��mol-1��֪������1mol CO����41.1kJ����������������������ﵽƽ��ʱ����1mol CO��������41.1kJ�������������������Ӧ���ۺ���ЧӦΪ���ȣ��ų�������=313.8kJ-41.1kJ=272.7kJ���ﵽƽ��ʱ��������ʵ����仯��Ϊ2mol����v(CH4)=
CO(g)+H2O(g) ��H2=41.1kJ��mol-1��֪������1mol CO����41.1kJ����������������������ﵽƽ��ʱ����1mol CO��������41.1kJ�������������������Ӧ���ۺ���ЧӦΪ���ȣ��ų�������=313.8kJ-41.1kJ=272.7kJ���ﵽƽ��ʱ��������ʵ����仯��Ϊ2mol����v(CH4)=![]() =0.1mol��L-1��min-1����������������CH4��ƽ��Ũ��Ϊ
=0.1mol��L-1��min-1����������������CH4��ƽ��Ũ��Ϊ![]() =2mol/L��CO2��ת��Ũ��Ϊ
=2mol/L��CO2��ת��Ũ��Ϊ![]() =3mol/L����CH4ѡ����=
=3mol/L����CH4ѡ����=![]() ��100%=
��100%=![]() ��100%=66.7%������ӦCO2(g)+4H2(g)
��100%=66.7%������ӦCO2(g)+4H2(g)![]() CH4(g)+2H2O(g)��ƽ�ⳣ��K=
CH4(g)+2H2O(g)��ƽ�ⳣ��K=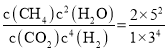 =
=![]() (��0.62)��
(��0.62)��
(3)����������������ȸ���Ӧ������Ϊ��ѡ���˵绯�������������Ǵ������нϸߵĴ����ԺͲ���ѡ���ԣ����������ⷴӦ������
�ڵ��ص������õ��ӣ�������ԭ��Ӧ��CO2��CԪ�صĻ��ϼ�Ϊ+4�ۣ������(CH3CH(OH)CH3)��CԪ�صĻ��ϼ�Ϊ-2�ۣ�����1mol�����(CH3CH(OH)CH3)ת��18mole-������ͼʾ����������������ĵ缫��ӦΪ3CO2+18H++18e-=CH3CH(OH)CH3+5H2O��

����Ŀ�����и��������У��ܴ��������Ҽ���(��ͨ��)X�Լ�������Ӧ�����ӷ���ʽ��Ӧ��ȷ����
ѡ�� | ������ | �Լ�X | ���ӷ���ʽ |
A | Fe3+��Al3+�� | ���������� |
|
B | ����Һ�У�Fe3+�� | ������ͭ�� | 2Fe3++Cu�T2Fe2++Cu2+ |
C | Na+��Ba2+�� | NaHSO4��Һ | H++ |
D | pH=1����Һ�У�Mg2+��Fe2+�� | ˫��ˮ | 2Fe2++H2O2+2H+�T2Fe3++2H2O |
A.AB.BC.CD.D