��Ŀ����
17������������������CH3CH2CH2CH2CH3
��CH3CH2CH2OH
��CH3CH2CH3
��CH3CHBrCH3
�ݣ�CH3��2-CH-CH=CH-CH3
��

��CH2OH-CHOH-CH2OH
�ࣨCH3��2-CH-COOH
��CH3CH2CH2CHO
���㰴Ҫ��������л���������з��࣬������ȷ�𰸵���������±��У�
| ���� | ϩ�� | ������ | ±���� | �� | ȩ | ���� | ֬���� |
| �٢� | �� | �� | �� | �ڢ� | �� | �� | �٢ۢ� |
���� ����ͼʾ���л����������ɼ��ṹ��ʽ�к��еĹ����Ž��з�����ɣ��籥����Ϊ����������̼̼˫������Ϊϩ�������б�����Ϊ��Ϊ�������ȣ����Ĺ�����Ϊ�ǻ������ǻ�ֱ���������������DZ�����һ�����������ȩ�Ĺ�����Ϊȩ��������Ĺ�����Ϊ�Ȼ�������֬���廯����������Ե�̼�⻯�������֬������
��� �⣺�������ڱ������������ж��ǵ����������������Т٢ۣ�
ϩ�������к���̼̼˫��������ϩ�����Тݣ�
������Ϊ�����к��б������������ڷ��������Тޣ�
±����������Ϊ±��ԭ�ӣ�����±�������Тܣ�
���Ĺ�����Ϊ�ǻ������ǻ�ֱ���������������DZ�����һ��������������ڴ����У��ڢߣ�
ȩ�Ĺ�����Ϊȩ��������ȩ���У��
����Ĺ�����Ϊ�Ȼ������������Ϊ���ࣻ
����֬���廯����������Ե�̼�⻯�������֬������������̼ԭ�Ӽ��������״��̼�ܣ������ſ������ɻ������������������������������Ϊ֬���������ֽṹ������Ҳ����֬��������Щ�����������ϲ�ͬ�ڷ���������ʮ������֬�������������֬������������֬�������Ϊ����������������������ܳƣ��٢ۢݣ�
�ʴ�Ϊ���٢ۣ��ݣ��ޣ��ܣ��ڢߣ��ᣬ�࣬�٢ۢݣ�
���� ���⿼�����л���ķ��������Ŀ�ѶȲ����漰�������Դ�ע��һЩ�л��ﺬ�ж��ֹ����ţ��ɴӸ������ŵĽǶȿ��ɲ�ͬ����л��ע��֬�����ķ����жϣ����ջ����ǹؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | �ƺ�ˮ��ӦNa+H2O�TNa++OH-+H2�� | |
| B�� | ��������������������Һ Al+2OH-�TAlO2-+H2�� | |
| C�� | ����������������Al+6H+�TAl3++3H2�� | |
| D�� | FeCl2��Һ�м�����ˮ��Cl2+2Fe2+�T2Fe3++2Cl- |
| A�� | ��ͬ�����£������顢�����顢������ķе��������� | |
| B�� | ����ױ���Ϊͬϵ�����ʹKMnO4������Һ��ɫ | |
| C�� | �ױ���Cl2�����µķ�Ӧ���Ҵ�������ķ�Ӧ���ڲ�ͬ���͵ķ�Ӧ | |
| D�� | �з�����ζ��C9H18O2�����������¼��ȿ�ˮ�������Է���������ͬ�������л������ϴ�������C9H18O2�Ľṹ��16�� |
| A�� | ����������ʢװ���Ũ���� | |
| B�� | ������������Һ��ȥ�����������Ĥ��Al2O3+2OH-�T2AlO2-+H2O | |
| C�� | ����ˮ���γɵ�Al��OH��3����������ˮ�������������ˮ�ľ��� | |
| D�� | ��������Ʒ���渲��������Ĥ�����ڲ������𱣻����ã������������������������ |
| A�� | O2+2H2O+4e-�T4OH- | B�� | 2H++2e-�TH2 | ||
| C�� | Fe-2e-�TFe2+ | D�� | 4OH--4e-�T2H2O |
| A�� | ����������ϡ�����У�FeS+2 H+�TFe2++H2S�� | |
| B�� | NH4HCO3���ڹ���NaOH��Һ�У�HCO3-+OH-�TCO32-+H2O | |
| C�� | ����������̼ͨ�뱽������Һ�У�C6H5O-+CO2+H2O�TC6H5OH+CO32- | |
| D�� | ����ʯ���ڴ����У�CaCO3+2CH3COOH�TCa2++2CH3COO-+CO2��+H2O |

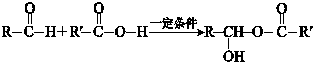

 ��
��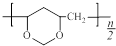 ��
��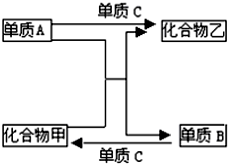 A��B��C�����ֶ�����Ԫ�صĵ��ʣ��ס��������ֳ����Ļ������Щ������͵���֮���������ͼ��ʾ�Ĺ�ϵ���ݴ��жϣ�
A��B��C�����ֶ�����Ԫ�صĵ��ʣ��ס��������ֳ����Ļ������Щ������͵���֮���������ͼ��ʾ�Ĺ�ϵ���ݴ��жϣ�