��Ŀ����
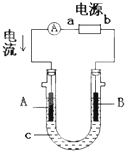
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��Ӧʽ ��
��X�������۲쵽�������� ��
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
����˵�������ʷ����ĵ缫��Ӧ����д����
��3����ҵƷ�������ص���Һ�к���ijЩ���������ʣ��������ӽ���Ĥ������ᴿ��������װ�������ӽ���Ĥ��ֻ����������ͨ�������乤��ԭ����ͼ��ʾ.
�ٸõ��۵�������Ӧʽ��____________________________��
��ͨ�翪ʼ������������ҺpH�����������ԭ��
___________________________________________________________________________��
�۳�ȥ���ʺ������������Һ����Һ����_________________����д��A����B����������
��1����2H++2e-=H2������2H2O+2e- =2H2��+2OH-���� ��Һ��ɺ�ɫ��
��2Cl-+2e-=Cl2�� �Ƿ�ʹʪ��KI-������ֽ������
��2����X��ͭ��1�֣��� Cu2++2e- =Cu�� ��Y����ͭ��1�֣���Cu-2e- = Cu2+
��3���٣�4OH--4e-=2H2O+O2�� ������������H+�ŵ磬�Ӷ��ƻ���ˮ�ĵ���ƽ�⣬ʹ��c��OH-������pH���ߡ� ��B �� 1�֣� ��������2�֣�
����:

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺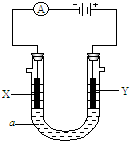 ��I��Li-SOCl2��ؿ����������������õ�صĵ缫���Ϸֱ�Ϊ﮺�̼�����Һ��LiAlCl4-SOCl2����ص��ܷ�Ӧ�ɱ�ʾΪ��4Li+2SOCl2=4LiCl+S+SO2��
��I��Li-SOCl2��ؿ����������������õ�صĵ缫���Ϸֱ�Ϊ﮺�̼�����Һ��LiAlCl4-SOCl2����ص��ܷ�Ӧ�ɱ�ʾΪ��4Li+2SOCl2=4LiCl+S+SO2��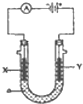 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������