��Ŀ����
����̼������ͭ�ȳ���Ԫ���ڻ������г����ֳ����ּ�̬��������-2��-1��+2��+4��+6�ȼ�̬����ЩԪ���ڻ���������������ҪӦ�á�
��1��31.2gþ��̼�۵Ļ������һ��������ǡ����ȫ��Ӧ���ټ�������ˮ���õ�40.6g��ɫ������ͬʱ�����ܶ�Ϊ1.4107g/L����״�����ı���ϩ�Ͳ�������X�Ļ�����塣
��þ��̼�۵ķ�Ӧ����Ļ�ѧʽΪ ______________ ��
��ԭ�������̼�۵����ʵ�������Ϊ ___________������С����ʾ������2λС����
��2��ij������������Ԫ����ɣ������������Ӻ����������ӡ�ȡ21.76g�ø����Σ�ƽ����Ϊ���ݡ�����һ���������������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ�����ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ���������˺�����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣
�ٸø����εĻ�ѧʽΪ_____________��
�ڸø����������������ӵ�������Ϊ _________________ ��
��3��ij����ѧ�Ҿ����ⷢ��һ���µĴŻ�������Ҫ�ɷ�ΪFe1-xS1+x�����ʲ���Fe��S��������֪�ôŻ������У���Ԫ��������������������Ϊ75%��ȡ5�� 73%�ĸôŻ������ýӴ��������ᣬ������¯�����յ������Ϊ4%���Ӵ����з�Ӧ��ת����Ϊ94%��SO3������Ч��Ϊ97%���������Ƶ�98.3%��Ũ�����������________________��������2λС����
��1��31.2gþ��̼�۵Ļ������һ��������ǡ����ȫ��Ӧ���ټ�������ˮ���õ�40.6g��ɫ������ͬʱ�����ܶ�Ϊ1.4107g/L����״�����ı���ϩ�Ͳ�������X�Ļ�����塣
��þ��̼�۵ķ�Ӧ����Ļ�ѧʽΪ ______________ ��
��ԭ�������̼�۵����ʵ�������Ϊ ___________������С����ʾ������2λС����
��2��ij������������Ԫ����ɣ������������Ӻ����������ӡ�ȡ21.76g�ø����Σ�ƽ����Ϊ���ݡ�����һ���������������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ�����ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ���������˺�����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣
�ٸø����εĻ�ѧʽΪ_____________��
�ڸø����������������ӵ�������Ϊ _________________ ��
��3��ij����ѧ�Ҿ����ⷢ��һ���µĴŻ�������Ҫ�ɷ�ΪFe1-xS1+x�����ʲ���Fe��S��������֪�ôŻ������У���Ԫ��������������������Ϊ75%��ȡ5�� 73%�ĸôŻ������ýӴ��������ᣬ������¯�����յ������Ϊ4%���Ӵ����з�Ӧ��ת����Ϊ94%��SO3������Ч��Ϊ97%���������Ƶ�98.3%��Ũ�����������________________��������2λС����
��1����MgC2��Mg2C3 ��0.63
��2����Cu5S(SO4)2 ��+1�۵�ͭ��+2�۵�ͭ=4:1
��3��6.08t
���������
��1���ٸ��������֪��̼���V��Ӧ�õ��Ļ�������ˮ��Ӧ�IJ������ϩC3H4������ݻ��������������ϼ۵Ĵ�����Ϊ0��ȷ�������к���Mg2C3������ϩC3H4����Է�������Ϊ40������ϩ�Ͳ�������X�Ļ�������״�����ܶ�Ϊ1.4107g/L����ƽ����Է�������Ϊ1.4107��22.4=31.6.˵��������������Է�������С��31.6��ֻ��C2H2��C2H4�����ݻ��������������ϼ۵Ĵ�����Ϊ0��������C2H2��ȷ�������л����к���MgC2��������C2H4��ȷ�������л����к���MgC�����ʵ����ȥ�����þ��̼�۵ķ�Ӧ����Mg2C3��MgC2���ٹ������õ���Mg(OH)2�����ʵ���Ϊn(Mg(OH)2)="m��M=" 40.6g�� 58g/mol =0.7mol.����ԭ31.2gþ��̼�۵Ļ���ﺬ�е�C�����ʵ���Ϊn(C)=(31.2g��0.7mol�� 24g/mol) ��12 g/mol=1.2mol�� ԭ�������̼�۵����ʵ�������Ϊ1.2mol��(0.7+1.2)mol=0.63.
��2������һ�����������ữ���Ȼ�����Һ�����ó����к�9.32g���ᱵ��֤������SO42-�������ʵ���Ϊn(SO42-)="9.32g��233g/mol=0.04mol;" ���ڶ�������������Ũ���ᣬ�ٵμ��������ᱵ��Һ����13.98g��ɫ����������13.98g>9.32g֤��ԭ�����л�����S2-������������������������SO42-����n(SO42-) (��)="13.98g��233g/mol=0.06mol;" n(S2-)="0.06-0.04=0.02mol." ����ɫ��Һ�м������ռ���Һ�����ˡ�ϴ�ӡ����գ���8.00g��ɫ���塣֤������CuԪ��n(Cu2+)=" 8.00g��80g/mol=0.1mol." n(Cu2+): n(S2-): n(SO42-)=0.1:0.02:0.04=5:1:2.���Ը��εĻ�ѧʽΪCu5S(SO4)2 �ڸ�����Cu�Ļ��ϼ���+1��+2���ּ�̬������+1�۵�Ϊx����+2�۵�Ϊ(5-x).���ݻ��������������ϼ۵Ĵ�����Ϊ0�ɵ�x+2(5-x)=2+4.���x=4����5-x=1.����m(Cu+):m(Cu2+)=4:1.
��3���ôŻ������У���Ԫ��������������������Ϊ75%�������������������Ϊ25%��n(Fe3+):n(Fe2+)=3:1.���ݻ��������������ϼ۵Ĵ�����Ϊ0����ȷ����������S��ԭ�Ӹ���Ϊ3��3��1��2=11.���Ըû�����Ļ�ѧʽΪFe4S11.���������֪���ת��Ϊ�����S�����ʵ���Ϊ{(5��106g��73%��(1��4%)��94%��97%)��576g/mol}��11=6.1��104mol.���Եõ���98.3%��Ũ����������ǣ�6.1��104mol��98g/mol����98.3%=6.08��106g="6.08t" ��

��ϰ��ϵ�д�
�����Ŀ
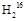 O��80 g
O��80 g 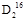 O�����ʵ���֮����������,����������������֮��������������,����������֮������������,���Ƿֱ���Na��Ӧʱ,���ų��������֮��(ͬ����)��������������,����֮������������������
O�����ʵ���֮����������,����������������֮��������������,����������֮������������,���Ƿֱ���Na��Ӧʱ,���ų��������֮��(ͬ����)��������������,����֮������������������