��Ŀ����
��֪Ag2SO4��KWΪ2.0��10-3��������Ag2SO4��������100 mLˮ�����պñ��ͣ��ù�����Ag+��SO42-Ũ����ʱ��仯��ϵ����ͼ������Ag2SO4��Һ��c(Ag+)��0.034��mol��L-1������t1ʱ����������ϵ�м���100��mL.��0.020��mol��L-1 Na2SO4��Һ������ʾ��ͼ�У�����ȷ��ʾt1ʱ�̺�Ag+��SO42-Ũ����ʱ��仯��ϵ���ǣ� ��
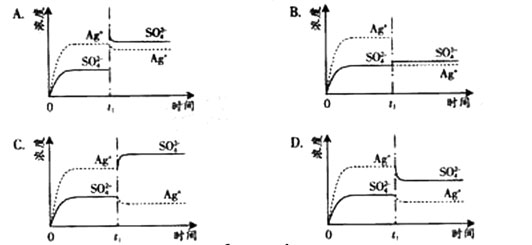

B
Ag2SO4�պ�Ϊ100ml�ı�����Һ,��Ϊc(Ag+)=0.034mol/L������c��SO42-��=0.017mol/L��������100ml 0.020mol/LNa2SO4��Һ��c��SO42-��=0.0185mol/L��c��Ag+��=0.017mol/L����ʱQ<Ksp�����ɼ����֪ѡB��

��ϰ��ϵ�д�
�����Ŀ

 )]2=KSP������KSPΪ��������Ϊ���¶���Mg(OH)2���ܶȻ����������ƶ�������þ�����������е��ܽ���ɴ�С��˳����
)]2=KSP������KSPΪ��������Ϊ���¶���Mg(OH)2���ܶȻ����������ƶ�������þ�����������е��ܽ���ɴ�С��˳����  AlCl3��Һ ��0.1mol��L
AlCl3��Һ ��0.1mol��L