题目内容
【题目】化学学习小组进行实验测定H2C2O4·xH2O 中x值。已知:M(H2C2O4)=90 g·mol-1
①称取 1.260 g 纯草酸晶体,将其酸制成 100.00 mL 水溶液为待测液;
②取25.00mL 待测液放入锥形瓶中,再加入适量稀H2SO4;
③用浓度为 0.05 000 mol·L-1的 KMnO4标准溶液进行滴定。
(1)请写出与滴定有关反应的离子方程式__________。
(2)某学生的滴定方式(夹持部分略去)如下,最合理的是__________(选填 a、b)。
a. 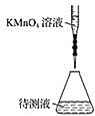 b.
b. 
(3)由图可知消耗KMnO4溶液体积为_______mL。

(4)滴定过程中眼睛应注视________。
(5)通过上述数据,求得x=______。以标准KMnO4溶液滴定样品溶液的浓度,未用标准KMnO4溶液润洗滴定管,引起实验结果______ (偏大、偏小或没有影响) 。
【答案】5H2C2O4+2MnO4-+6H+=10CO2↑+2Mn2++8H2O b 20.00 锥形瓶中颜色变化 2 偏小
【解析】
(1)H2C2O4溶液和酸性KMnO4溶液反应生成二氧化碳、锰离子和水;
(2)据高锰酸钾具有强氧化性,盛放在酸式滴定管中来选择;
(3)根据滴定管的结构、精确度以及测量原理来解答;
(4)滴定时眼睛应注视锥形瓶中颜色变化;
(5)据此6H++5H2C2O4+2MnO4-=2Mn2++10CO2↑+8H2O离子方程式来计算;分析错误操作对V[KMnO4(aq)]的影响,结合反应原理进行误差分析。
(1)草酸具有还原性,高锰酸钾具有强氧化性,把草酸转化成CO2,高锰酸钾被还原成Mn2+,根据化合价升降法,进行配平,因此离子反应方程式为5H2C2O4+2MnO4-+6H+=10CO2↑+2Mn2++8H2O。故答案为:6H++5H2C2O4+2MnO4-=2Mn2++10CO2↑+8H2O;
(2)高锰酸钾溶液具有强氧化性,能腐蚀橡胶管,应用酸式滴定管盛放高锰酸钾溶液,b正确;故答案为:b;
(3)滴定前刻度为0.90 mL,滴定后刻度是20.90 mL,消耗高锰酸钾的体积为(20.90-0.90) mL=20.00 mL;故答案为:20.00;
(4) 滴定时眼睛应注视锥形瓶中颜色变化,以便及时判断滴定的终点,
故答案为:锥形瓶中颜色变化
(5)6H++5H2C2O4+2MnO4-=2Mn2++10CO2↑+8H2O
5 2
n(H2C2O4) 0.02L×0.05mol/L
解得n(H2C2O4)=0.0025mol
则100mL溶液的H2C2O4物质的量为0.0025mol×![]() =0.01mol,M(H2C2O4·xH2O )=1.26g÷0.01mol=126g/mol,则x=
=0.01mol,M(H2C2O4·xH2O )=1.26g÷0.01mol=126g/mol,则x=![]() =2;
=2;
未用标准KMnO4溶液润洗滴定管,溶液被稀释,造成V[KMnO4(aq)]偏大,则H2C2O4物质的量偏大,H2C2O4·xH2O的摩尔质量偏小,x偏小。
故答案为:2;偏小。

 英才计划同步课时高效训练系列答案
英才计划同步课时高效训练系列答案【题目】以高纯H2为燃料的质子交换膜燃料电池具有能量效率高、无污染等优点,但燃料中若混有CO将显著缩短电池寿命。
(1)以甲醇为原料制取高纯H2是重要研究方向。甲醇水蒸气重整制氢主要发生以下两个反应:
主反应:CH3OH(g)+H2O(g)![]() CO2(g)+3H2(g) △H=+49 kJmol-1
CO2(g)+3H2(g) △H=+49 kJmol-1
副反应:H2(g)+CO2(g)![]() CO(g)+H2O(g) △H=+41 kJmol-1
CO(g)+H2O(g) △H=+41 kJmol-1
①甲醇在催化剂作用下裂解可得到H2和CO,则该反应的化学方程式为_________________________,既能加快反应速率又能提高CH3OH平衡转化率的一种措施是_________________________。
②分析适当增大水醇比(nH2O∶nCH3OH)对甲醇水蒸气重整制氢的好处_________________________。
③某温度下,将nH2O∶nCH3OH =1∶1的原料气充入恒容密闭容器中,初始压强为p1,反应达到平衡时总压强为p2,则平衡时甲醇的转化率为_________________________。(忽略副反应)
(2)工业常用CH4 与水蒸气在一定条件下来制取H2,其原理为:
CH4(g)+H2O(g)=CO(g)+3H2(g) ΔH=+203kJ·mol-1
①该反应的逆反应速率表达式为; V逆=k·c(CO)·c3(H2),k为速率常数,在某温度下,测得实验数据如表:
CO浓度(mol·L-1) | H2浓度(mol·L-1) | 逆反应速率(mol·L-1·min-1) |
0.05 | C1 | 4.8 |
c2 | C1 | 19.2 |
c2 | 0.15 | 8.1 |
由上述数据可得该温度下,上述反应的逆反应速率常数k 为__________L3·mol-3·min-1。
②在体积为3L的密闭容器中通入物质的量均为3mol 的CH4和水蒸气,在一定条件下发生上述反应,测得平衡时H2的体积分数与温度及压强的关系如图所示,则压强Pl_____P2(填“大于”或“小于”)温度T3_______T4(填“大于”或“小于”);压强为P1时,在N点; v正_______v逆(填“大于”或“小于”或“等于”)。求N点对应温度下该反应的平衡常数 K=_____________________。

【题目】三氧化二镍(Ni2O3)是一种灰黑色无气味有光泽的块状物,易碎成细粉末,常用于制造高能电池。工业上以金属镍废料生产NiCl2,继而生产Ni2O3的工艺流程如下:

下表列出了相关金属离子生成氢氧化物沉淀的pH(开始沉淀的pH按金属离子浓度为1.0 mol·L-1计算)。
氢氧化物 | Fe(OH)3 | Fe(OH)2 | Al(OH)3 | Ni(OH)2 |
开始沉淀的pH | 1.8 | 5.8 | 3.0 | 7.1 |
沉淀完全的pH | 3.2 | 8.8 | 5.0 | 9.2 |
(1)为了提高金属镍废料浸出的速率,在“酸浸”时可采取的措施有①适当升高温度;②搅拌;③________等。
(2)酸浸后的酸性溶液中含有Ni2+、Cl-,另含有少量Fe2+、Fe3+、Al3+等。沉镍前需加Na2CO3控制溶液pH范围为____________________。
(3)从滤液A中可回收利用的主要物质是Na2CO3和________。
(4)“氧化”生成Ni2O3的离子方程式为__________________________________。
(5)工业上用镍为阳极,电解0.05~0.1 mol·L-1 NiCl2溶液与一定量NH4Cl组成的混合溶液,可得到高纯度、球形的超细镍粉。当其他条件一定时,NH4Cl的浓度对阴极电流效率及镍的成粉率的影响如图所示,则①NH4Cl的浓度最好控制为__________________________。
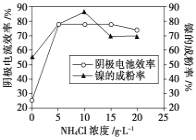
②当NH4Cl的浓度大于15g·L-1时,阴极有气体生成,导致阴极电流效率降低,写出相应的电极反应式:________________________。
(6)如果在“沉镍”步骤把Na2CO3改为加草酸,则可以制得草酸镍晶体(NiC2O4·2H2O)。草酸镍晶体在热空气中干燥脱水后在高温下煅烧三小时,可以制得Ni2O3,同时获得混合气体。草酸镍晶体受热分解的化学方程式为___________________________________。