��Ŀ����
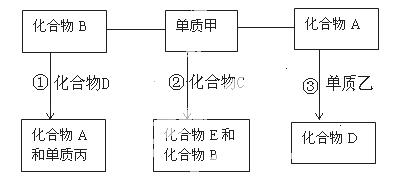
����A��G����ѧ��ѧ�г��������ʣ���Щ��������ͼ��ʾ��ת����ϵ(���ַ�Ӧ�������û���г�)������A��B��F����һ����ͬ��Ԫ�أ�C��D��G����һ����ͬ��Ԫ�أ�F���д��ԣ�GΪ��ɫ�ǽ������ʡ�

(1)����A�ܽ������ᣬȻ�����KSCN��Һ����Һ����ɫ���ٵμӼ�����ˮ�� ��Һ��ΪѪ��ɫ���ɴ���֪A�Ļ�ѧʽΪ__________��
��Һ��ΪѪ��ɫ���ɴ���֪A�Ļ�ѧʽΪ__________��
(2)��Ӧ�۵Ļ�ѧ����ʽ��_______________________ _______________________��
_______________________��
(3)д���������ʵĻ�ѧʽ�� E__________��F__________��
E__________��F__________��
(4)��C��һ�����壬���¶�Ϊ1 100 ���ij�̶��ݻ����ܱ������з�����Ӧ��A(s)��C(g)B(s)��D(g)����H��a kJ��mol��1(a>0)�����¶���ƽ�ⳣ��K��0.263��������1 mol B�������յ�����________(ѡ����ڡ��������ڡ���С�ڡ�)a kJ�������������A����C��ת����________(ѡ����ߡ��������䡱���͡�)����������ѹǿ������ʱ��仯����÷�Ӧ__________(ѡ��ﵽ������δ�ﵽ����һ���ﵽ��)��ѧƽ��״̬���÷�Ӧ�ﵽ��ѧƽ��״̬ʱ����c(C)��0.100 mol��L��1����c(D)��________mol��L��1��
�ŷ�ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
��ӦFe(s)+H2O(g) FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
| 700�� | 900�� | |
| K1 | 1��47 | 2��15 |
| K2 | 2��38 | 1��67 |
�ٷ�ӦCO2(g) + H2(g) CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g)
CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g) CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
�����ж�CO2(g) + H2(g) CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
A��������ѹǿ���� B�����������c(CO)����
C��v��(H2)= v��(H2O) D��c(CO)= c(CO2)
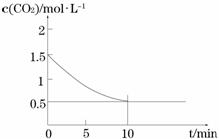 ��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g)
��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
�� �������·�Ӧ��ƽ�ⳣ��Ϊ ��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ_________mol��L��1��
�����д�ʩ����ʹƽ��ʱ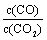 �������______������ţ���
�������______������ţ���
A�������¶� B������ѹǿ
C������һ������CO2 D���ټ���һ��������


 2C���磩�ﵽƽ��ı�־��
2C���磩�ﵽƽ��ı�־��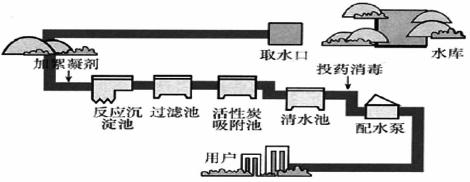
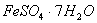 �dz��õ�������������ˮ����������__________�������ѧʽ����
�dz��õ�������������ˮ����������__________�������ѧʽ����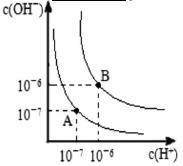
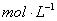 ��NaOH��Һ�У���ˮ�������c��H+��=________
��NaOH��Һ�У���ˮ�������c��H+��=________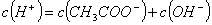
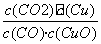
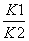
 ������������⣺
������������⣺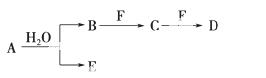
 _______________��
_______________��