��Ŀ����
����Ŀ�������Լ���RMgX�����л���Ӧ�е�һ����Ҫ�Լ��������Ʒ�Ϊ�� ��RΪ������XΪ±�أ���
��RΪ������XΪ±�أ���
������ϩ���ʵ�������ϳ������춡��![]() �Ĺ������£���Ӧ����û���г�����
�Ĺ������£���Ӧ����û���г�����

�Իش��������⣺
��1�����������У����ڣ���������ӳɷ�Ӧ����________________________������ţ���
��2��A�����������ŵ�������____________��C�����������ŵ�������____________��
��3����ϵͳ��������F��������������________________________��
��4��д��E�Ľṹ��ʽ________________________��
��5����Ӧ�ڵĻ�ѧ����ʽ____________________________________��
��6����Ӧ�ߵĻ�ѧ����ʽ____________________________________��
��7��B��������Һ��Ӧ�ķ���ʽ____________________________________��
��8��д��C��������ͬ����л�����칹��____________��____________��
���𰸡� �٢ܢ� �ǻ� �Ȼ� 2-���� CH3CH2MgX 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3CH2CH��OH��CH3+CH3COOH
2CH3CHO+2H2O CH3CH2CH��OH��CH3+CH3COOH![]() CH3COOCH(CH3)CH2CH3+H2O CH3CHO+2Ag��NH3��2OH
CH3COOCH(CH3)CH2CH3+H2O CH3CHO+2Ag��NH3��2OH![]() CH3COONH4+3NH3��+2Ag+H2O HCOOCH3 HOCH2CHO
CH3COONH4+3NH3��+2Ag+H2O HCOOCH3 HOCH2CHO
����������ϩ��ˮ�����ӳɷ�Ӧ����AΪCH3CH2OH���Ҵ���������������BΪCH3CHO����ȩ��һ������������Ӧ����CΪCH3COOH����ϩ��HX�����ӳɷ�Ӧ����DΪCH3CH2X��D��Mg�����������·�����Ӧ����EΪCH3CH2MgX��E����ȩ�����ӳɷ�Ӧ��ˮ������FΪCH3CH2CH��OH��CH3�������ᷢ��������Ӧ�õ������춡������
��1���������Ϸ�����֪���������У����ڣ���������ӳɷ�Ӧ���Ǣ٢ܢޡ���2��A���Ҵ����������������ŵ��������ǻ���C�����ᣬ�������������ŵ��������Ȼ�����3��FΪCH3CH2CH��OH��CH3����������2-��������4��E�Ľṹ��ʽΪCH3CH2MgX����5����Ӧ�����Ҵ�����������Ӧ������ȩ����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����6����Ӧ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CH2CH��OH��CH3+CH3COOH
2CH3CHO+2H2O����6����Ӧ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CH2CH��OH��CH3+CH3COOH![]() CH3COOCH(CH3)CH2CH3+H2O����7��B��������Һ��Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
CH3COOCH(CH3)CH2CH3+H2O����7��B��������Һ��Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH![]() CH3COONH4+3NH3��+2Ag+H2O����8��C�����ᣬC��������ͬ����л�����칹��ΪHCOOCH3��HOCH2CHO��
CH3COONH4+3NH3��+2Ag+H2O����8��C�����ᣬC��������ͬ����л�����칹��ΪHCOOCH3��HOCH2CHO��

����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��a��b��c��d��e��ԭ��������������a��b�����������������Ԫ�أ�c�ǵؿ��к�������Ԫ�أ�d��bͬ�壬e2+���ӵ�3d�������9�����ӡ��ش��������⣺
��1��c��d����Ԫ���γɵĻ�����ͳ�ƹ�ʯ����ͨ��______________����������ᾧ�κ����ε����ִ�����̬��c�ĵ����Ų�ͼΪ_______________________��
��2��A��B�����������ֳ������л��A����CaCO3��Ӧ�������ڳ�����ˮ����B�����е�̼ԭ����Ŀ��A����ͬ���ɷ���������Ӧ��A�д��ڻ�ѧ����������______��
A.���Ӽ� B.���Լ� C.�Ǽ��Լ� D.�Ҽ� E.�м�
B���ӹ�������̼ԭ�ӵĹ���ӻ�������____��
��3���á�>����<����գ�
��һ������ | �۵� |
b___d | dc2����___d���� |
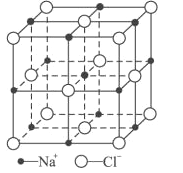
��4��c��e����Ԫ�ؿ��γ�һ�ְ뵼����ϣ���ѧʽΪe2c���������������ڲ����ĸ�cԭ�ӣ�����cԭ��λ�����ĺͶ��㣬��þ�������____��eԭ�ӣ�eԪ��λ��Ԫ�����ڱ���_______����
��5����e2+�����ε�ˮ��Һ�м�������İ�ˮ���ɵõ�����ɫ����Һ�������Ҵ�����������ɫ���壬����д������������ӷ���ʽ___________________��
��6��e����Ϊ�����������壬��ԭ�Ӱ뾶Ϊrcm���侧���ⳤΪa nm����e���ʵ��ܶ�Ϊ__________g��cm-3����ռ������ʵļ���ʽΪ_______________________��