��Ŀ����
����Ŀ��������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У�����������NaClO2����������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��
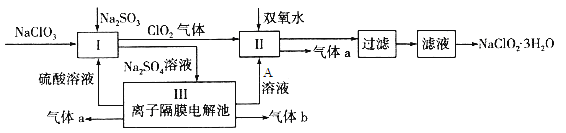
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�������£�Ksp��FeS��=6.3��10-18��Ksp��CuS��=6.3��10-36��Ksp��PbS��=8��10-28
�ۢ�װ���е����������Һ����������������ͬʱ�������ᣬ��������������ͬʱ�����������ơ�
��1�� I�з�����Ӧ�����ӷ���ʽΪ ____________��
��2������Һ�еõ�NaClO2��3H2O������������������ ����д�������
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ��������������NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
���� | HClO2 | HF | H2CO3 | H2S |
Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�������£����ʵ���Ũ����ȵ�NaClO2��NaF��NaHCO3��Na2S������Һ��pH�ɴ�С��˳��Ϊ ���û�ѧʽ��ʾ����
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij����� �������£������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��ϵ�е�S2-��Ũ��Ϊ ��
��4����װ��������������a�����Ϊ1.12L����״��������ת�Ƶ��ӵ����ʵ���Ϊ________��
���𰸡���1��2ClO3-��2H+��SO32-=2ClO2����SO42-��H2O��2��bdc
��3����Na2S>NaHCO3>NaF>NaClO2����CuS��6.3��10-13mol/L��4��0.2mol
��������
�����������1�� I�������ƽ�������������Ϊ�����ƣ���������ԭΪ�������ȣ�������Ӧ�����ӷ���ʽΪ2ClO3-��2H+��SO32-=2ClO2����SO42-��H2O��
��2���Ʊ�������Ҫ�����IJ��������Ǽ�����������ȴ�ᾧ�����ˡ�ϴ�ӵ�����ѡbdc��
��3�����������������ݿ�֪������ƽ�ⳣ��Խ������Խǿ������ƽ�ⳣ��ԽС������Խ�����γ��κ�Ϊǿ�������Σ�����Խ��������Һ�ļ���Խǿ��pHԽ��������Һ��pH�ɴ�С��˳��ΪNa2S>NaHCO3>NaF>NaClO2������Ũ�ȵ�Cu2����Fe2����Pb2�����ӣ��μ�Na2S��Һ�����������ij�����Ksp��С�ģ���ΪCuS�������һ�����ӳ�����ȫʱ����Ksp��FeS����6.3��10-18��c��Fe2+��c��S2-����10-5c��S2-�������c��S2-����![]() mol/L��6.3��10-13mol/L��
mol/L��6.3��10-13mol/L��
��4����װ���ǵ����������Һ����������a�ĵ缫ͬʱ���������������˵���õ缫�������������ŵ�������������������Ϊ1.12L����״���������ʵ���Ϊ1.12L��22.4L/mol��0.05mol�����ת�Ƶ��ӵ����ʵ���Ϊ0.05mol��4��0.2mol��

����Ŀ����ȥ������е����ʣ�������Ϊ����������ѡ�Լ��ͷ��뷽������ȷ����
����� | �����Լ� | ���뷽�� | |
A | �屽���壩 | NaOH��Һ | ��Һ |
B | ��������ϩ�� | ����KMnO4 | ϴ�� |
C | �ױ������ӣ� | ��ˮ | ���� |
D | �����飨�Ҵ��� | NaOH��Һ | ��Һ |
A. A B. B C. C D. D
