��Ŀ����
����Ŀ��ij��ѧ��ȤС����ʵ����ͨ������ͼ��ʾװ���Ʊ�Na2S2O3��

��1��װ��a��ʢװNa2SO3���������������________��
��2��װ��b��������_______��
��3��ʵ�������,װ��d�е�������NaOH��Na2CO3����������________(�ѧʽ)��
��4��װ��c�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
��5��ʵ�������װ��c����Һ��������Ҫ��Na2S2O3��������Na2CO3��Na2SO3�ȳɷݡ�Ϊ��֤��Na2CO3�Ĵ��ڣ��������ʵ�顣��ѡ�Լ�:AƷ����Һ��B���Ը��������Һ��C BaCl2��Һ��D����ʯ��ˮ��E ϡ����
�� ȡC����Һ�������μ�����___________(���Լ���ţ���
�� ������������������ͨ��________(���Լ���š���ʵ������ͽ���Ϊ_______��
���𰸡���1��Բ����ƿ��1�֣���2��������ȫƿ����������2�֣�
��3��Na2SO3��2������4��Na2CO3+2Na2S+4SO2=3Na2S2O3+CO2��2����
��5����E��2�֣���B��A��D KMnO4��Һ��ɫ��dz��Ʒ����Һ����ɫ������ʯ��ˮ����ǣ���C��Һ�к���Na2CO3����4�֣�
��������
�����������1�����������Ĺ����ص��֪��װ��a��ʢװNa2SO3���������������Բ����ƿ��
��2������װ�ÿ�֪��aװ�����Ʊ�SO2�ģ�cװ�����Ʊ�Na2S2O3�ģ���װ��b��������������ȫƿ����������
��3������Ӧ����SO2ʣ�࣬SO2��NaOH��Һ��Ӧ����Na2SO3��ˮ������ʵ�������,װ��d�е�������NaOH��Na2CO3����������Na2SO3��
��4��װ��c����Na2CO3��Na2S�Ļ��Һ��ͨ��SO2������Ӧ����Na2S2O3��CO2����Ӧ�Ļ�ѧ����ʽΪNa2CO3+2Na2S+4SO2=3Na2S2O3+CO2��
��5��װ��c����Һ��������Ҫ��Na2S2O3��������Na2CO3��Na2SO3�ȳɷݣ�Ҫ֤��Na2CO3�Ĵ��ڣ���Ҫ�ų�SO2����ĸ��ţ����岽�����ȼ�������ϡ���ᣬ��Ӧ����SO2��CO2���壬Ȼ�������Ը��������Һȥ��SO2����Ʒ����Һ���SO2�Ƿ���������ó���ʯ��ˮ����CO2�Ĵ��ڡ������� ȡC����Һ�������μ�����E���� ������������������ͨ��B��A��D��ʵ������ͽ���ΪKMnO4��Һ��ɫ��dz��Ʒ����Һ����ɫ������ʯ��ˮ����ǣ���C��Һ�к���Na2CO3��

����Ŀ������ij�ֹ��ԣ��ɽ�CO2��SO2��Ϊһ����������������У�Ҳ����ͬ����������ǣ�������
A.CaCO3
B.SO3
C.CuO
D.KMnO4
����Ŀ��������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У�����������NaClO2����������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��
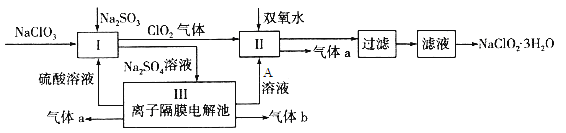
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�������£�Ksp��FeS��=6.3��10-18��Ksp��CuS��=6.3��10-36��Ksp��PbS��=8��10-28
�ۢ�װ���е����������Һ����������������ͬʱ�������ᣬ��������������ͬʱ�����������ơ�
��1�� I�з�����Ӧ�����ӷ���ʽΪ ____________��
��2������Һ�еõ�NaClO2��3H2O������������������ ����д�������
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ��������������NaClO2��Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
���� | HClO2 | HF | H2CO3 | H2S |
Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�������£����ʵ���Ũ����ȵ�NaClO2��NaF��NaHCO3��Na2S������Һ��pH�ɴ�С��˳��Ϊ ���û�ѧʽ��ʾ����
��Na2S�dz��õij�������ij��ҵ��ˮ�к��е�Ũ�ȵ�Cu2+��Fe2+��Pb2+���ӣ��μ�Na2S��Һ�����������ij����� �������£������һ�����ӳ�����ȫʱ��������Ũ��Ϊ10-5mol��L-1����ʱ��ϵ�е�S2-��Ũ��Ϊ ��
��4����װ��������������a�����Ϊ1.12L����״��������ת�Ƶ��ӵ����ʵ���Ϊ________��
����Ŀ�����и��������У����ܰ�ͼʾ����������ʾһ����ɣ���ϵ�ת������
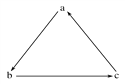
ѡ�� | a | b | c |
A | Fe | FeCl3 | FeCl2 |
B | H2SO4 | SO2 | SO3 |
C | Si | SiO2 | H2SiO3 |
D | HNO3 | NO | NO2 |
A. A B. B C. C D. D
