��Ŀ����
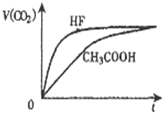 ��ͬ�¶ȣ�Ũ�Ⱦ�Ϊ0.1mol/L���������������Һ����HF��Һ����CH3COOH��Һ����NaHCO3��Һ����֪�� �١��ڷֱ���ۻ�ϣ�ʵ���ò�����CO2���������v����ʱ�䣨t���ı仯��ͼ��ʾ������˵����ȷ���ǣ�������
��ͬ�¶ȣ�Ũ�Ⱦ�Ϊ0.1mol/L���������������Һ����HF��Һ����CH3COOH��Һ����NaHCO3��Һ����֪�� �١��ڷֱ���ۻ�ϣ�ʵ���ò�����CO2���������v����ʱ�䣨t���ı仯��ͼ��ʾ������˵����ȷ���ǣ�������| A������������Һ�У�c�� OH-����С���ۣ��ڣ��� |
| B���������ԵıȽϣ�CH3COOH��HF��H2CO3 |
| C����Ӧ��������������Һ�У�c��CH3COO-����c��F-�� |
| D���ٺ� �۷�Ӧ������������Һ�У�c��F-��+c��HF���T0.10mol/L |
���㣺���������ˮ��Һ�еĵ���ƽ��
ר�⣺����ƽ������Һ��pHר��
������A��HF�����Ա�CH3COOHǿ������Һ��c��H+���ϴ����������ӵ�Ũ�Ƚ�С����NaHCO3��Һ��ǿ���������ʽ��ˮ��̶ȴ��ڵ���̶ȣ���Һ�ʼ��ԣ�
B��ͼ���з�����HF�����Ա�CH3COOHǿ���������ԵıȽϣ�HF��CH3COOH��H2CO3��
C��ͼ���з�����HF�����Ա�CH3COOHǿ������������ˮ��̶�c��CH3COO-����c��F-����
D��HF��Һ��NaHCO3��Һ��Ӧ���ɷ�������Һ�������غ���㣻
B��ͼ���з�����HF�����Ա�CH3COOHǿ���������ԵıȽϣ�HF��CH3COOH��H2CO3��
C��ͼ���з�����HF�����Ա�CH3COOHǿ������������ˮ��̶�c��CH3COO-����c��F-����
D��HF��Һ��NaHCO3��Һ��Ӧ���ɷ�������Һ�������غ���㣻
���
�⣺A��HF�����Ա�CH3COOHǿ������Һ��c��H+���ϴ����������ӵ�Ũ�Ƚ�С����NaHCO3��Һ��ǿ���������ʽ��ˮ��̶ȴ��ڵ���̶ȣ���Һ�ʼ��ԣ�����c�� OH-����С���ۣ��ڣ��٣���A��ȷ��
B��ͼ���з�����HF�����Ա�CH3COOHǿ���������ԵıȽϣ�HF��CH3COOH��H2CO3����B����
C��ͼ���з�����HF�����Ա�CH3COOHǿ������������ˮ��̶�c��CH3COO-����c��F-������Һ����������Ũ��ԽС������c��CH3COO-����c��F-������C����
D��HF��Һ��NaHCO3��Һ��Ӧ���ɷ�������Һ�������غ�c��F-��+c��HF���T
=0.05mol/L����D����ѡA��
B��ͼ���з�����HF�����Ա�CH3COOHǿ���������ԵıȽϣ�HF��CH3COOH��H2CO3����B����
C��ͼ���з�����HF�����Ա�CH3COOHǿ������������ˮ��̶�c��CH3COO-����c��F-������Һ����������Ũ��ԽС������c��CH3COO-����c��F-������C����
D��HF��Һ��NaHCO3��Һ��Ӧ���ɷ�������Һ�������غ�c��F-��+c��HF���T
| 0.10mol/L��1L |
| 1L+1L |
���������⿼����������ʵĵ���ƽ��Ӧ�ã�ͼ�����������ˮ�ĵ���Ӱ�����أ���Ҫ��������ǿ���ıȽϣ�����ˮ���Ӧ�ã��������Һ��Ԫ���غ��Ӧ�ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
���з�Ӧ����ȡ����Ӧ���ǣ�������
| A���Ҵ���Ũ�����ϼ�����170�� |
| B������Һ���Ϻ����������м |
| C���ױ������Ը��������Һ��� |
| D����ϩͨ�����Ը��������Һ�� |
����˵���У���ȷ���ǣ�������
| A��HCl��H2SO4����ͬ������������ˮ��Һ�����ʱ�����в�ͬ�������ӣ��������ǵĻ�ѧ����������ͬ |
| B�������������һ�������⡢��Ԫ�� |
| C���ܵ�����������ӵĻ�����һ�������� |
| D����Cu��OH��2���뵽����ʯ����Һ������ˮ�У���Һ����ɫ |
�������ʵ������������ԭ������������ȷ���ǣ�������
| A���ڡ���˾ƥ�ֵĺϳɡ�ʵ���У��Ѵ������������ľ�����г��ˣ����Ҵ�ϴ�Ӿ���1��2�Σ�Ȼ����ˣ�������ת�Ƶ��������ϣ���������������������� |
| B����������Ĵ�С��ᾧ�����йأ����ʵ��ܽ��ԽС������Һ��Ũ��Խ�ߣ����ܼ��������ٶ�Խ�죬�����ľ�����ԽϸС |
| C�������еı�ʪ�������ͣ�������������ͭ��Һ���ݲ��������ɫ���������� |
| D�����Թ��н�һ������Ũ���ᡢŨ����ͱ���ϣ��þƾ���ֱ�Ӽ��ȼ����ӣ������Ƶ������� |
������֤��Ԫ�صķǽ����Ա���Ԫ��������ʵ�ǣ�������
����������ڼ������������γ�H2S��H2S����300�����ҷֽ⣮�����������ڵ�ȼ����������� �Ȼ��⣬�Ȼ�����ѷֽ�
������������Һ�е�����ˮ�е���������
���������ֱ���ͭ������Ӧ������FeS��Cu2S��FeCl3��CuCl2
�ܸ����ᣨHClO4��������ǿ�����ᣮ
����������ڼ������������γ�H2S��H2S����300�����ҷֽ⣮�����������ڵ�ȼ����������� �Ȼ��⣬�Ȼ�����ѷֽ�
������������Һ�е�����ˮ�е���������
���������ֱ���ͭ������Ӧ������FeS��Cu2S��FeCl3��CuCl2
�ܸ����ᣨHClO4��������ǿ�����ᣮ
| A���٢ڢۢ� | B��ֻ�Т٢� |
| C��ֻ�Тڢۢ� | D��ֻ�Тۢ� |
��һ������л��軯��й��飬���ǵ�������������ƣ�����˵���д�����ǣ�������
| A������ķ���ʽ����ͨʽSinH2n+2��ʾ |
| B�����飨SiH4��û�м����ȶ� |
| C��������ȼ�գ�����SiO2��H2O |
| D��������ܶȱȼ�����ܶ�С |
��2L�ܱ������У���һ�������·���A��g��+3B��g��?2C��g������10s�ڷ�Ӧ��A��Ũ����1mol/L����0.4mol/L����ͣ�C��Ϊ��������
| A��0.06mol/��L?s�� |
| B��0.12mol/��L?s�� |
| C��0.6mol/��L?s�� |
| D��1.2mol/��L?s�� |
����̫���ܵ����Ҫ�ߴ��ȵĹ裬��ҵ���Ƹߴ��賣�����·�Ӧʵ��
��Si��s��+3HCl��g��
SiHCl3��g��+H2��g�� ��SiHCl3+H2
Si+3HCl
������������Ӧ�����������У�������ǣ�������
��Si��s��+3HCl��g��
| ||
| ||
������������Ӧ�����������У�������ǣ�������
| A��������Ӧ�����û���Ӧ |
| B����Ӧ�ڲ����û���Ӧ |
| C����ҵ���Ƶøߴ�������ڼ����оƬ |
| D��������Ӧ����������ԭ��Ӧ |