��Ŀ����
����Ŀ��150��ʱ������ͼ��ʾ�ĺ�ѹ�����м���4LN2�� H2�Ļ�����壬�ڴ��������³�ַ�Ӧ(����������Բ���)������Ӧ���ȡ���Ӧ��ָ���ԭ�¶ȡ�ƽ������������Ϊ3.4L���������������ͬ����������������ܶ�Ϊ5��
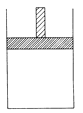
(1)��Ӧǰ���������V(N2):V(H2)=______����Ӧ��ƽ���V(NH3)=__L���÷�Ӧ��N2ת����Ϊ___��
(2)��ƽ���������г���0.2mol��NH3��һ��ʱ���Ӧ�ٴδﵽƽ�⣬�ָ���150��ʱ��ô˹����д����������6.44 kJ����������ش𣺳���NH3ʱ�����������ܶȽ�__________(����������������С������������)���ڴﵽƽ��Ĺ����У����������ܶȽ�____(����������������С������������)����Ӧ���´�ƽ����������������������ܶȽ�______5(����>������<���� �� = ��)
���𰸡� 1:3 0.6 30% ���� ��С =
��������������������⿼�黯ѧƽ��ļ��㣬���º�ѹ�µĵ�Чƽ����Ӱ�컯ѧƽ��������Լ����������ı仯��
��1����ԭN2�����Ϊx����ԭH2�����Ϊ4-x��ת����N2���Ϊy���ں��º�ѹ���������֮�ȵ������ʵ���֮�ȣ���������ʽ
N2 + 3H2![]() 2NH3
2NH3
V����ʼ����L�� x 4-x 0
V��ת������L�� y 3y 2y
V��ƽ�⣩��L�� x-y 4-x-3y 2y
����x-y��+��4-x-3y��+2y=3.4L��y=0.3L��ƽ����������������ͬ����������������ܶ�Ϊ5��ƽ����������ƽ����Է�������Ϊ5![]() 2=10�����������غ��28x+2��4-x��=10
2=10�����������غ��28x+2��4-x��=10![]() 3.4��x=1L����Ӧǰ���������V��N2����V��H2��=1L����4L-1L��=1:3����Ӧ��ƽ���V��NH3��=2
3.4��x=1L����Ӧǰ���������V��N2����V��H2��=1L����4L-1L��=1:3����Ӧ��ƽ���V��NH3��=2![]() 0.3L=0.6L��N2��ת����Ϊ0.3L
0.3L=0.6L��N2��ת����Ϊ0.3L![]() 1L
1L![]() 100%=30%��
100%=30%��
��2�����ڸ�����Ϊ���º�ѹ���������������ܶ���ƽ��Ħ�����������ȣ�ƽ����ٳ���0.2mol��NH3������NH3��Ħ��������17g/mol������ƽ��ʱƽ��Ħ��������10g/mol��������NH3ʱ���������ܶȽ���������0.2molNH3���ü���һ�ߵ������Ϊ0.1molN2��0.3molH2����ԭƽ�����ʼ��Ӧ���ʳɱ�������ﵽ����ƽ����ԭƽ��Ϊ���º�ѹ�µĵ�Чƽ�⣬�����´ﵽƽ��ʱ��������ƽ��Ħ��������Ϊ10g/mol�������ڴﵽƽ��Ĺ����У����������ܶȽ���С�����´ﵽƽ��ʱ������������������ܶȲ������Ե���5��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�