��Ŀ����
18�� ̼Ԫ���ǹ����л���Ļ���Ԫ�أ�
̼Ԫ���ǹ����л���Ļ���Ԫ�أ���1��д��̼Ԫ�غ�������Ų�ʽ1s22s22p2����Ԫ�����ڱ��е�λ�õڶ����ڵڢ��壮
��2��������ˮ������Է������������ܷе�ȴ�кܴ��࣬ԭ����ˮ���Ӽ���γ������
��3���������顢��ϩ����Ȳ��̼ԭ���ӻ���ʽ����sp3����ϩsp2����Ȳsp1��
��4����֪CO2�����ṹ��ͼ���˾������ڷ��Ӿ��壬������ÿ��CO2������Χ���ڵ�CO2������12������֪�侧���߳�Ϊa cm��NA��ʾ�����ӵ����������ܶ�Ϊ$\frac{176}{{N}_{A}{a}^{3}}$ g/cm3��
���� ��1��Cԭ�Ӻ�����6�����ӣ����ݵ����Ų�ʽ�������ܼ���Ԫ�������������ڵ��Ӳ������������������������ӣ�
��2������Ĵ����ܹ�ʹ���ʵ��۷е����ߣ�
��3�����ݿռ乹��ȷ��ԭ���ӻ����ͣ�
��4��������̼�����Ƕ�����̼����ͨ�����Ӽ���������϶��ɣ�
CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ��ݴ˴��⣻
����CO2�����ṹ�ɼ��㺬�еķ������������ʵ����������������ܶȹ�ʽ�������ܶȣ�
��� �⣺��1����Ԫ��Ϊ6��Ԫ�أ�ԭ�Ӻ�����6�����ӣ�������Ų�ʽΪ��1s22s22P2��������2�����Ӳ㣬�������4�����ӣ����������ڱ���λ�ã��ڶ����ڵڢ��壻
�ʴ�Ϊ��1s22s22p2���ڶ����ڵڢ��壻
��2��ˮ�����е���ԭ�ӷǽ�����ǿ������ˮ���Ӽ���γ������ʹˮ�ķе�������ߣ�
�ʴ�Ϊ��ˮ���Ӽ���γ������
��3�����顢��ϩ����Ȳ�Ŀռ乹�ͷֱ����������塢ƽ���͡�ֱ���ͣ�����sp3��sp2��sp1��
�ʴ�Ϊ������sp3����ϩsp2����Ȳsp1��
��4��������̼�����Ƕ�����̼����ͨ�����Ӽ���������϶������ڷ��Ӿ��壻
�ɱ��Ƿ��Ӿ��壬CO2����λ��������Ķ���������ϣ��Զ����ϵ�CO2����Ϊ�����������������CO2���ӷֲ�����ö���������12����������ϣ�
����CO2�����ṹ�ɼ�������еķ�����Ϊ8��1/8+6��1/2=4�������ʵ�����$\frac{4}{{N}_{A}}$��������$\frac{4��44}{{N}_{A}}$�����ܶ�=$\frac{m}{��}$
=$\frac{176}{{N}_{A}{a}^{3}}$��
�ʴ�Ϊ�����Ӿ��壻12��$\frac{176}{{N}_{A}{a}^{3}}$��
���� ���⿼���˺�����ӵ��Ų����ɡ�ԭ�ӵ��ӻ����������Ľṹ����Ϥԭ�ӽṹ�����幹�������ռ�ṹ�ǽ���ؼ�����Ŀ�ѶȽϴ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| ��� | ʵ������ | ʵ��Ŀ�� |
| A | ��ʢ��10��0.1 mol•L-1AgNO3��Һ���Թ��еμ�0.1mol•L-1 NaCl��Һ���������г������ɣ��������еμ�0.1mol•L-1 Na2S��Һ | ֤��AgCl��ת��Ϊ�ܽ�ȸ�С��Ag2S |
| B | ȡij��Һ���������������ữ���Ȼ�����Һ�����ְ�ɫ���� | ֤������Һ��һ�����д�����SO42- |
| C | ��ij±�����м���NaOH��Һ������һ��ʱ�����AgNO3��Һ | ����±�����е�±��ԭ�� |
| D | ���еμ��������ᣬ������������ͨ�뱽������Һ | ֤�����ԣ����̼����� |
| A�� | A | B�� | B | C�� | C | D�� | D |

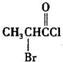 ��
��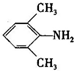 �Ǻϳ�ʩ������
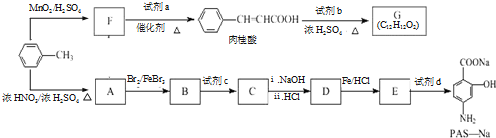
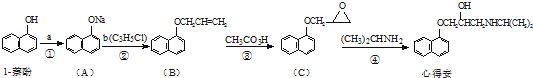
�Ǻϳ�ʩ������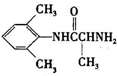 ��һ�ֿ�����ʧѧҩ����м��壬�ֱ�����ͼ��ʾ·�ߺϳɣ�
��һ�ֿ�����ʧѧҩ����м��壬�ֱ�����ͼ��ʾ·�ߺϳɣ�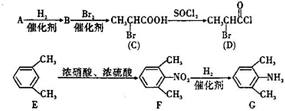 ����֪������-NH2�����ǰ�����-NH-�����м��ԣ�
����֪������-NH2�����ǰ�����-NH-�����м��ԣ�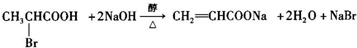 ��
�� ��
�� ��
��


 ��
�� ��
�� ��
��
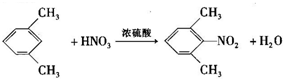
 ����F����һ��������Ļ�ѧ����ʽΪ��
����F����һ��������Ļ�ѧ����ʽΪ�� +HNO3��Ũ��$��_{��}^{Ũ����}$
+HNO3��Ũ��$��_{��}^{Ũ����}$ +H2O�����㻯����F�������ǶԱ������ᣮ
+H2O�����㻯����F�������ǶԱ������ᣮ