��Ŀ����
��ˮռ�����ܴ�ˮ����97%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ����Ҳ���Ե�������Ȼ����Ƶ��ƺ��������÷�Ӧ����ʽΪ________________________��
��2�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�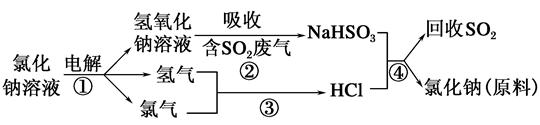
��д����Ӧ�Ļ�ѧ����ʽ��
��__________����__________����__________��
��1��2NaCl�����ڣ�  2Na��Cl2��
2Na��Cl2��
��2����NaOH��SO2=NaHSO3
��H2��Cl2 2HCl
2HCl
��NaHSO3��HCl=NaCl��SO2����H2O
����

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
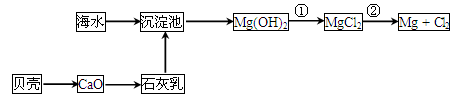
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д���(�۵㣭39�棬�е�356��)�������ء��缫�ȵ���Ҫԭ�ϣ���ʷ�����á����ճ�ɰ������ȡ����Ŀǰ��ҵ���ƴֹ���һ������ͼ���¡�
������������
A�������ճ�ɰ���������е���ת�Ƶķ������Ŀ�ɱ�ʾΪ�� |
| B����ɰ�������Ƽ��ȷ�Ӧʱ��CaSO4Ϊ�������� |
| C��ϴ�Ӵֹ�����5%���������5%������ |
| D����ѹ�����Ŀ���ǽ����ķе㣬��߷���Ч�� |
��������ۺ����ü������ڽ�Լ��Դ���������ڱ���������ʵ�������÷Ͼɻ�ͭ(Cu��Zn�Ͻ𣬺���������Fe)�Ʊ���������(CuSO4��5H2O)��������ZnO���Ʊ�����ͼ���£� 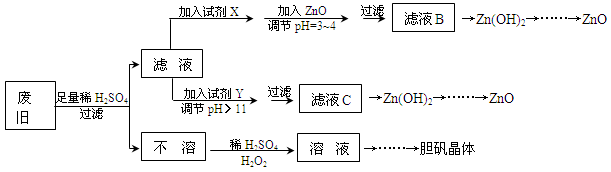
��֪��Zn���������������Al����������������ƣ�pH��11ʱZn(OH)2������NaOH��Һ����[Zn(OH)4]2�����±��г��˼������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L��1����)��
| | Fe3�� | Fe2�� | Zn2�� |
| ��ʼ������pH | 1.1 | 5.8 | 5.9 |
| ������ȫ��pH | 3.0 | 8.8 | 8.9 |
��ش��������⣺
��1���Լ�X������__________����������____________________��
��2������ZnO����pH=3��4��Ŀ����____________________��
��3���ɲ�����������ҺD�Ļ�ѧ����ʽΪ______________________________��
��4������ҺD�Ƶ��������������Ҫ����������______________________________��
��5�������Լ�����ΪY�Լ�����______��
A��ZnO B��NaOH C��Na2CO3 D��ZnSO4
������ҺC����μ�������ֱ���������������������______________________________��
��6���ⶨ��������Ĵ���(��������I��������Ӧ������������)��ȷ��ȡ0.5000g��������������ƿ�У�������ˮ�ܽ⣬�ټ������KI����0.1000mol��L��1Na2S2O3����Һ�ζ����յ㣬����Na2S2O3����Һ19.40mL����֪�������ζ������е����ӷ���ʽ���£�
2Cu2����4I��
 2CuI(��ɫ)����I2��I2��2S2O32��
2CuI(��ɫ)����I2��I2��2S2O32�� 2I����S4O62��
2I����S4O62���ٵ�������Ĵ���Ϊ_______________��
���ڵζ������о���ҡ��(��Һ���⽦)��ƿ��������õĴ��Ƚ���__________(�ƫ�ߡ�����ƫ�͡����䡱)��
�����й��������ֵ������������(����)��
| A�����ӱ�Ҫ��Ԫ�أ����Ƹֲĵ���֯�ṹ������ |
| B���ʵ����������еĺ�̼������ȥ���������� |
| C������衢�̡����ȺϽ�Ԫ�ص����ɷֲ���ȥ��ˮ�е��� |
| D����ȥ�����еķǽ���Ԫ�� |


 �������ѱ䣬�����⣨2H�������˾۱䣬��LiH��Ұ����������ܺ�ˮɹ��
�������ѱ䣬�����⣨2H�������˾۱䣬��LiH��Ұ����������ܺ�ˮɹ��
 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��