��Ŀ����
����Ŀ���л���X��ҩ����м��壬����һ�ֺϳ�·�����¡�

��֪��RNH2+ +H2O
+H2O
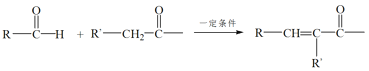
��1��A��֧����A�к��еĹ�����������___��
��2��A���������IJ������£�
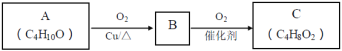
Aת��ΪB�Ļ�ѧ����ʽ��___��
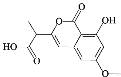
��3��MΪ���㻯�����ṹ��ʽ��___��
��4��M��N�Ļ�ѧ����ʽ��___����Ӧ������___��
��5������˵������ȷ����___��
a��1molD��NaOH��Һ��Ӧʱ���������2molNaOH
b��E��һ�������¿����ɸ߷��ӻ����� 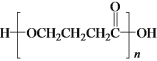
c��F�ܷ���������Ӧ����ȥ��Ӧ
��6��Q�Ľṹ��ʽ��___��
��7������ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�A��д���ϳ�·��___���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
���𰸡��ǻ� 2CH3CH2CH2CH2OH+O2![]() 2CH3CH2CH2CHO+2H2O
2CH3CH2CH2CHO+2H2O ![]()
![]() +HNO3
+HNO3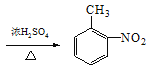 +H2O ȡ����Ӧ c
+H2O ȡ����Ӧ c 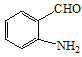 CH2=CH2
CH2=CH2![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]() CH3CH=CHCHO
CH3CH=CHCHO![]() CH3CH2CH2CH2OH
CH3CH2CH2CH2OH
��������
A�ɾ������������õ�C�����ݣ�2����������������Ϣ����֪AΪ1-������BΪ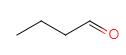 ��CΪ
��CΪ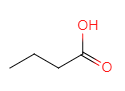 ��C��D����ȡ����Ӧ��DΪ
��C��D����ȡ����Ӧ��DΪ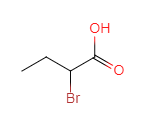 ��D��ˮ�⡢�ữ��õ�E��EΪ
��D��ˮ�⡢�ữ��õ�E��EΪ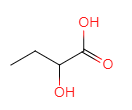 ��E��������������F
��E��������������F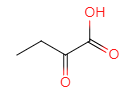 ��M�IJ����Ͷ�Ϊ4���������ղ���X�Ľṹ����֪MΪ�ױ������ݺ��������֪��M��N����������λ��һȡ����Ӧ��NΪ
��M�IJ����Ͷ�Ϊ4���������ղ���X�Ľṹ����֪MΪ�ױ������ݺ��������֪��M��N����������λ��һȡ����Ӧ��NΪ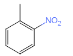 ��N����������ԭ��õ�����Q
��N����������ԭ��õ�����Q ��
��
��������������֪��
��1��AΪ1-������������Ϊ�ǻ���
��2��Aת��ΪBΪ��������Ӧ����ѧ����ʽ��2CH3CH2CH2CH2OH+O2![]() 2CH3CH2CH2CHO+2H2O��
2CH3CH2CH2CHO+2H2O��
��3��M�Ľṹ��ʽΪ![]() ��
��
��4��M��NΪȡ����Ӧ����ѧ����ʽ��![]() +HNO3
+HNO3 +H2O��
+H2O��
a��1molD��NaOH��Һ��Ӧʱ���������2molNaOH��a��ȷ��
b��E��һ�������¿����ɸ߷��ӻ����� 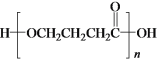 ��b��ȷ��
��b��ȷ��
c��F�ܷ���������Ӧ����������ȥ��Ӧ��c����
�ʴ�ѡc��
��6��Q�Ľṹ��ʽ�� ��
��
��7����ϩˮ�����Ҵ����Ҵ�����Ϊ��ȩ��������Ŀ��Ϣ��2����ȩ���ӷ�����Ӧ�������![]() ��
��![]() ����������������Ӧ������1-����������ΪCH2=CH2
����������������Ӧ������1-����������ΪCH2=CH2![]() CH3CH2OH
CH3CH2OH![]() CH3CHO
CH3CHO![]() CH3CH=CHCHO
CH3CH=CHCHO![]() CH3CH2CH2CH2OH��
CH3CH2CH2CH2OH��