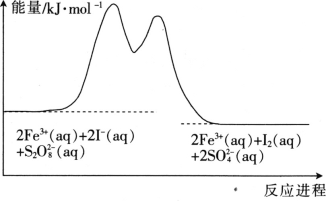
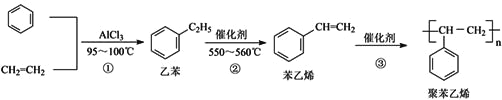
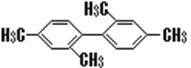
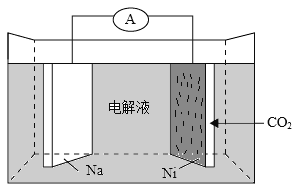
��Ŀ����
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
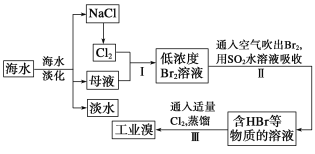
��1���Ӻ�ˮ�п��Եõ�ʳ�Ρ�Ϊ�˳�ȥ�����е�Ca2����Mg2����SO42-����ɳ���ɽ���������ˮ��Ȼ�������������������ٹ��ˣ��ڼӹ���NaOH��Һ���ۼ��������ᣬ�ܼӹ���Na2CO3��Һ���ݼӹ���BaCl2��Һ����ȷ�IJ���˳����___��
A���٢ܢڢݢ� B���ܢ٢ڢݢ� C���ڢݢܢ٢� D���ڢܢݢ٢�
��2����������ѻ��Br2����������ֽ�Br2��ԭΪBr������Ŀ��Ϊ___��
��3���������SO2ˮ��Һ����Br2ͬʱ��H2SO4���ɣ������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ___���ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������___��
���𰸡�C ������ Br2+SO2+2H2O=4H++SO42-+2Br- ǿ����豸�ĸ�ʴ
��������
(1)��ȥ�����е�Ca2����Mg2����SO42-����ɳ����ʹ��Na2CO3��Һ��Ca2+��NaOH��Һ��Mg2+��BaCl2��Һ��SO42-�����������ӵļ��������ǹ����ģ������迼�ǹ������ӵĴ�����������Ba2+��ʹ��CO32-����������Na2CO3��ҺӦ��BaCl2��Һ֮�������Һ�У�����Ӧ�ڹ���֮�������Һ�У��Է������ܽ⡣�ɴ˱��ȷ����ȷ�IJ���˳��
��2����������ѻ��Br2����Ũ�Ⱥ�С����������ȡ�����Բ�������ֽ�Br2��ԭΪBr������Ҫ��Ϊ�˸����塣
��3���������SO2ˮ��Һ����Br2ͬʱ��HBr��H2SO4���ɣ��йط�Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++SO42-+2Br-���ɴ˷�Ӧ��֪������������ǿ�ᣬ�ḯʴ�豸���������dz����������⣬�ڹ�ҵ������Ӧ�������Ҫ���⡣
(1)��ȥ�����е�Ca2����Mg2����SO42-����ɳ����ʹ��Na2CO3��Һ��Ca2+��NaOH��Һ��Mg2+��BaCl2��Һ��SO42-�����������ӵļ��������ǹ����ģ������迼�ǹ������ӵĴ�����������Ba2+��ʹ��CO32-����������Na2CO3��ҺӦ��BaCl2��Һ֮�������Һ�У�����Ӧ�ڹ���֮�������Һ�У��Է������ܽ⡣�ɴ˱��ȷ����ȷ�IJ���˳��Ϊ�ڢݢܢ٢ۣ���ΪC��
��2����������ѻ��Br2����Ũ�Ⱥ�С����������ȡ�����Բ�������ֽ�Br2��ԭΪBr������Ҫ��Ϊ�˸����壻��Ϊ�������壻
��3���������SO2ˮ��Һ����Br2��ͬʱ��HBr��H2SO4���ɣ��йط�Ӧ�����ӷ���ʽΪBr2+SO2+2H2O=4H++SO42-+2Br-���ɴ˷�Ӧ��֪������������ǿ�ᣬ�ḯʴ�豸���������dz����������⣬�ڹ�ҵ������Ӧ�������Ҫ���⡣��Ϊ��Br2+SO2+2H2O=4H++SO42-+2Br-��ǿ����豸�ĸ�ʴ��