��Ŀ����
����Ŀ��1911�꣬��ѧ�ҷ��ֹ���4.2K����ʱ����ͻȻ�������㡪�������³����ԡ�1986�꣬��ѧ���ַ�����Nb3Ge��23K�¾��г�������1987��2�£������ͼ������߶�����������Һ������(�е�77 K)�ĸ��³����壬�侧����ͼ��ʾ��Ԫ�����ΪBa-Y-Cu-O(�ٽ��¶�93 K)���ƶ��˹��ʸ��³����о���������Ժʿ���2016��ȹ�����߿�ѧ��������
�ش��������⣺
��1����Nbλ�ڵ������ڣ�Nb����Χ�����Ų�ʽΪ4d45s1��Nbλ��_______��
��2�����й���GeԪ��������ȷ����______(������ѡ����ѡ��)
A��Ge����������������Ϊ���۾��� B��Ge����p���Ĺ��ɽ���
C��Ge�ĵ�һ�����ܱ�As��Se��ҪС D��Ge�ĵ縺�Ա�C��
��3��Ge(CH3)2Cl2���ӵ�����ԭ��Ge���ӻ���ʽ��______________
��4��NH3Ҳ����������������_______(����ڡ���С�ڡ�)109��28�䣬NH3�ķе�(239.6 K)����N2�е����Ҫԭ����___________________________
��5��ͼʾ���ϵ����뻯ѧʽ(��λʱ)Ϊ___________________����Y(��)Ԫ�صĻ��ϼ�Ϊ+3����Cu��ƽ�����ϼ�Ϊ_______
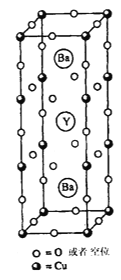
��6������ͭ���������������ܶѻ����侧����Cuԭ�ӵ��������Ϊa cm������ͭ�ľ����ܶ�Ϊ�� g/cm3������٤������ΪNA����ͭ�����ԭ������Ϊ________ (ֻ��һ��ϵ������a���ѡ�NA��ʾ)��
���𰸡�VBA Csp3С��NH3���Ӽ��������N2���Ӽ����������ʹNH3�ķе����Ba2YCu3O9+11/3![]() a3��NA��0.707a3��NA
a3��NA��0.707a3��NA
��������
��1����Nbλ�ڵ������ڣ�Nb����Χ�����Ų�ʽΪ4d45s1�������ڱ���ds������5������е�������λ�ڵ������ڣ�4d ����4�����Ӻ�5s����һ��������������VB������2��A��Geλ��Ԫ�����ڱ�����Ԫ����ǽ���Ԫ�صķֽ��߸���������������������Ϊ���۾��壬ѡ��A��ȷ��B��Ge����p�����������ڹ��ɽ�����ѡ��B����C��ͬ����Ԫ�ش����ҵ�һ������������As��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬����һ�����ܽϸߣ����һ������As>Se>Ge��ѡ��C��ȷ��D��ͬ������ϵ��·ǽ����Լ����縺�Լ�������Ge�ĵ縺�Ա�CС��ѡ��D����ѡAC����3��Ge(CH3)2Cl2���ӵ�����ԭ��Ge�ļ۲���Ӷ���4�����ӻ���ʽ��sp3����4��NH3Ҳ���������������ӹ����������Σ������С��109��28�䣬NH3�ķе�(239.6 K)����N2�е����Ҫԭ����NH3���Ӽ��������N2���Ӽ����������ʹNH3�ķе��������5�������ڲ�����2����ԭ�ӡ�1��Yԭ�ӣ����Ϻ���8��ͭԭ�ӡ�20��Oԭ�ӣ����㺬��8��ͭԭ�ӣ����ĺ���8��Oԭ�ӣ���3��ͭԭ�ӡ�9��Oԭ�ӣ�ͼʾ���ϵ����뻯ѧʽ(��λʱ)ΪBa2YCu3O9����Y(��)Ԫ�صĻ��ϼ�Ϊ+3����Cu��ƽ�����ϼ�Ϊ![]() ����6������ͭ���������������ܶѻ����侧���к���4��ͭԭ�ӣ�Cuԭ�ӵ��������Ϊa cm�����߳�Ϊ
����6������ͭ���������������ܶѻ����侧���к���4��ͭԭ�ӣ�Cuԭ�ӵ��������Ϊa cm�����߳�Ϊ![]() ������ͭ�ľ����ܶ�Ϊ�� g/cm3������٤������ΪNA������
������ͭ�ľ����ܶ�Ϊ�� g/cm3������٤������ΪNA������ ����ͭ�����ԭ������Ϊ
����ͭ�����ԭ������Ϊ![]() a3��NA��
a3��NA��

����Ŀ�������й�ʵ������ͽ��ͻ���۶���ȷ����
ѡ�� | ʵ����� | ʵ������ | ���ͻ���� |
A | ������NO2 ���ܱղ������������ˮ�� | ����ɫ���� | ��Ӧ2NO2 N2O4 �� |
B | ����������ˮ�ֱ����FeCl2��Һ��NaI��Һ��,�ٷֱ�μ�CCl4�� | �²�ֱ����ɫ���Ϻ�ɫ | ��ԭ��:I-��Br-��Fe2+ |
C | ij������������,������ɫ��ζ����,ͨ������ʯ��ˮ | �а�ɫ�������� | �ü�����̼��� |
D | ��̼��������ȷֽ����������ͨ��ij��Һ | ��Һ����ǣ�����ͨ������壬������ʧ | ����Һ������������Һ |
A. AB. BC. CD. D
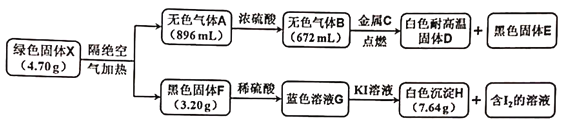
����Ŀ���������ҹ��������������������2.5�����µ�ϸ������(PM2.5)�ǵ�������������������������������е�CO��SO2�������������Ⱦ�����ͨ��������ѧ��Ӧ����PM2.5�����
��1�� ��CaSO4����O2��ȼ��CO��Ӧ����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ������ȿ����ȼ��Ч�ʣ����ܵõ��ϴ���CO2���Ա��ڱ���������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
����CaSO4(s)��4CO(g)==CaS(s)��4CO2(g) ��H1����189.2 kJ��mol-1
����CaSO4(s)��CO(g)==CaO(s)��CO2(g)��SO2(g)�� ��H2����210.5 kJ��mol-1
����CO(g)==![]() C(s)��
C(s)��![]() CO2(g) ��H3����86.2 kJ��mol-1
CO2(g) ��H3����86.2 kJ��mol-1
��Ӧ2CaSO4(s)��7CO(g)==CaS(s)��CaO(s)��6CO2(g)��C(s)��SO2(g)����H��_________________
��2����֪��CO����CO2�Ļ�ѧ����ʽΪCO��O2![]() CO2��O��������Ӧ����Ϊv��=K����c(CO) ��c(O2)���淴Ӧ����Ϊv��=K����c(CO2) ��c(O)��K����K��Ϊ���ʳ�������2500 K����K��=1.21��105 L��s-1��mol-1��K��=3.02��105 L��s-1��mol-1������¶���������Ӧ��ƽ�ⳣ��KֵΪ________(����С�����һλС��)��
CO2��O��������Ӧ����Ϊv��=K����c(CO) ��c(O2)���淴Ӧ����Ϊv��=K����c(CO2) ��c(O)��K����K��Ϊ���ʳ�������2500 K����K��=1.21��105 L��s-1��mol-1��K��=3.02��105 L��s-1��mol-1������¶���������Ӧ��ƽ�ⳣ��KֵΪ________(����С�����һλС��)��
��3���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������Ӧ��C(s)��2NO(g)![]() N2(g)��CO2(g)����H=Q kJ��mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2(g)����H=Q kJ��mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ��(min) Ũ��(mol��L-1) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
��0��10 min�ڣ�NO��ƽ����Ӧ����v(NO)=___________________________________��
��30 min��ֻ�ı�ijһ��������Ӧ����ƽ�⣬�����ϱ������жϸı������������____(ѡ����ĸ)��
a������һ�����Ļ���̿ b��ͨ��һ������NO c���ʵ���С��������� d��������ʵĴ���
����30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ5��3��3����Q_____0 (������������������������)��
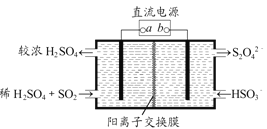
��4��������ͼ��ʾ���װ��(�缫��Ϊ���Ե缫)Ҳ������SO2�������������ų�����Һ����NO2�����Դb�����ӵĵ缫�ĵ缫��ӦʽΪ____________________________________��
��5��NO2��һ�������¿�ת��ΪNH4NO3��NH4NO2����ͬ�¶�������Ũ��NH4NO3��NH4NO2������Һ�����NH4NO2��Һ��c(NH4+)��С���������ܵ�ԭ��________________________��
����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g����H2��g��![]() CO��g����H2O��g����
CO��g����H2O��g����
�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��1���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK =__________________��
��2���÷�ӦΪ__________��Ӧ�������ȡ����ȡ�����
��3�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������_______________��
a��������ѹǿ���� b����������� c��CO������
c��������H2�������棨H2O�� d��c��CO2����c��CO��
��4��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c��CO2����c��H2����c��CO����c��H2O�������жϴ�ʱ���¶�Ϊ________����
����Ŀ������ʵ�����������ͽ��۾���ȷ����
ѡ�� | ʵ����� | ���� | ���� |
A | �����pH=2��HX��HY������ֱ�������������Ӧ������ˮ���ռ����� | HX�ų����������ҷ�Ӧ���ʿ� | ���ǿ����HX��HY |
B | ��2 mL 0.1 mol/L Na2S��Һ�е�2��0.1 mol/L ZnSO4��Һ���ٵ�2��0.1 mol/L CuSO4 | �����ɰ�ɫ�����������ɺ�ɫ���� | �ܶȻ�(Ksp)��ZnS>CuS |
C | ��FeCl3��KSCN�����Һ�У���������KCl�Ĺ��� | ��Һ��ɫ��dz | FeCl3+3KSCN |
D | �����£���pH�Ʒֱ�ⶨ����NaA��Һ�ͱ���NaB��Һ��pH | pH��NaA��NaB | ���������ԣ�HA��HB |
A. A B. B C. C D. D