��Ŀ����
����Ŀ����ijNaOH��Һ��ͨ��CO2�����õ���ҺM����CO2ͨ������IJ�ͬ����ҺM�����Ҳ��ͬ������M�м������ᣬ�������������V��CO2���������������V��HCl���Ĺ�ϵ��ͼ��ʾ�������з������жϲ���ȷ���ǣ�����CO2���ܽ⣩
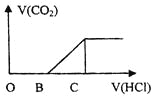
A.��OB=0�����γ���ҺM�����������ӷ���ʽΪOH-+CO2=![]()
B.��OB=BC������ҺMΪ![]() ��Һ
��Һ
C.��OB��BC������ҺM�д������ڵ�������Ϊ![]() ��
��![]()
D.��3OB=BC������ҺM��![]()
���𰸡�C
��������
��NaOH��Һ��ͨ��CO2�����ܷ����ķ�Ӧ��CO2+2OH��=![]() +H2O��CO2+OH��=
+H2O��CO2+OH��=![]() ����M��Һ�еμ����ᣬ�漰�ķ�Ӧ������H++OH��=H2O��
����M��Һ�еμ����ᣬ�漰�ķ�Ӧ������H++OH��=H2O��![]() +H+=
+H+=![]() ��
��![]() +H+=H2O+CO2����
+H+=H2O+CO2����
A.��OB=0����M��Һ�еμ����ᣬ�����������壬���������ӷ���ʽΪ![]() +H+=H2O+CO2����˵��MΪNaHCO3��Һ�����γ�M�Ĺ����з��������ӷ���ʽΪCO2+OH��=
+H+=H2O+CO2����˵��MΪNaHCO3��Һ�����γ�M�Ĺ����з��������ӷ���ʽΪCO2+OH��=![]() ����A��ȷ��
����A��ȷ��
B.��OB=BC����OB�����ĵ�������BC�����ĵ�������ȣ�OB�η�����Ӧ�����ӷ���ʽΪ![]() +H+=
+H+=![]() ����M��ҺΪNa2CO3��Һ����B��ȷ��
����M��ҺΪNa2CO3��Һ����B��ȷ��
C.��OB>BC����OB�η����ķ�Ӧ��H++OH��=H2O��![]() +H+=
+H+=![]() ������ҺM�д������ڵ�������ΪOH����
������ҺM�д������ڵ�������ΪOH����![]() ����C����
����C����
D.��3OB=BC���ɸ���![]() +H+=
+H+=![]() ��
��![]() +H+=H2O+CO2�����м��㣬
+H+=H2O+CO2�����м��㣬
OB�Σ�![]() + H+ =
+ H+ = ![]() ��
��
1mol 1mol 1mol
BC��![]() + H+ = H2O+CO2��
+ H+ = H2O+CO2��
3mol 3mol
M��Һ��n(NaHCO3)=3mol��1mol=2mol��n(Na2CO3)=1mol����n(NaHCO3)=2n(Na2CO3)������c(NaHCO3)=2c(Na2CO3)����D��ȷ��
��ѡC��

 ����������ϵ�д�
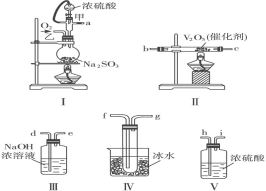
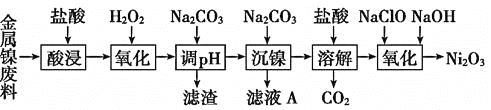
����������ϵ�д�����Ŀ������������![]() ��һ�ֻҺ�ɫ����ζ�й���Ŀ�״������ϸ��ĩ��������������ܵ�ء���ҵ���Խ���������
��һ�ֻҺ�ɫ����ζ�й���Ŀ�״������ϸ��ĩ��������������ܵ�ء���ҵ���Խ���������![]() ����������������������
����������������������![]() ���̶�����
���̶�����![]() �Ĺ����������£�
�Ĺ����������£�
�±��г�����ؽ������������������������![]() ��ʼ������pH����������Ũ��Ϊ
��ʼ������pH����������Ũ��Ϊ![]() ����
����![]() ��
��
�������� |
|
|
|
|
��ʼ������pH |
|
|
|
|
������ȫ��pH |
|
|
|
|
(1)Ϊ����߽��������Ͻ��������ʣ����������ʱ�ɲ�ȡ�Ĵ�ʩ��______
A. �����¶� ![]() ����
���� ![]() ���������Ũ��
���������Ũ�� ![]() �������гɷ�ĩ
�������гɷ�ĩ
(2)������������Һ�м���![]() ��Ŀ�ģ�__________������ǰ���
��Ŀ�ģ�__________������ǰ���![]() ������ҺpH��ΧΪ______�����õ���������Ҫ�ɷ���_______��
������ҺpH��ΧΪ______�����õ���������Ҫ�ɷ���_______��
(3)����ҺA�пɻ������õ���Ҫ������![]() ��________��
��________��
(4)������������![]() �����ӷ���ʽΪ__________________________��
�����ӷ���ʽΪ__________________________��
(5)��ҵ������Ϊ���������![]() ��Һ��һ����
��Һ��һ����![]() ��ɵĻ����Һ���ɵõ��ߴ��ȡ����εij�ϸ���ۡ�����������һ��ʱ��
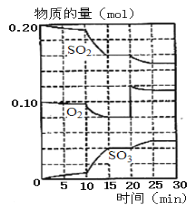
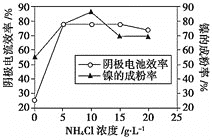
��ɵĻ����Һ���ɵõ��ߴ��ȡ����εij�ϸ���ۡ�����������һ��ʱ��![]() ��Ũ�ȶ���������Ч�ʼ����ijɷ��ʵ�Ӱ����ͼ��ʾ����
��Ũ�ȶ���������Ч�ʼ����ijɷ��ʵ�Ӱ����ͼ��ʾ����![]() ��Ũ����ÿ���Ϊ_________��
��Ũ����ÿ���Ϊ_________��
����Ŀ��Na��Al��Fe��Cu����ѧ��ѧ����Ҫ�Ľ���Ԫ�ء����ǵĵ��ʼ��仯����֮���кܶ�ת����ϵ���±��������ʲ��ܰ���ͼ(��������ʾһ�����)��ϵ�ת������
ѡ�� | A | B | C | D |
|
a | Na | Al | Fe | Cu | |
b | NaOH | Al2O3 | FeCl3 | CuO | |
c | NaCl | Al(OH)3 | FeCl2 | CuCl2 |
A.AB.BC.CD.D
����Ŀ����A��B��C��D����ǿ����ʣ�������ˮ�е���ʱ�ɲ����������ӣ�ÿ������ֻ��һ���������һ����ظ��� ��ʾ��CH3COONH4��ҺΪ����
������ | Na+��Ba2+��NH4+ |
������ | CH3COO����Cl����OH����SO42�� |
��֪����A��C��Һ��pH������7��A��B����Һ��ˮ�ĵ���̶���ͬ��
��C��Һ��D��Һ����ʱֻ���ɰ�ɫ������B��Һ��C��Һ����ʱֻ���ɴ̼�����ζ�����壬A��Һ��D��Һ���ʱ����������
��1��A��______ ��B��_______ ��
��2��25��ʱ��0.1mol��L-1B��Һ��pH=a����B��Һ��c(H+)��c(NH3��H2O)= _______���ú�a�Ĺ�ϵʽ��ʾ����
��3����������������ʵ���Ũ�ȵ�B��Һ��C��Һ��ϣ���Ӧ����Һ�и�������Ũ���ɴ�С��˳��Ϊ__________________��
��4����һ�����0.005 mol��L-1��C��Һ�У�����һ�������0.00125 mol��L-1������ʱ�������Һ��pH=11������Ӧ����Һ���������C��Һ����������֮�ͣ���C��Һ�������������� ____________��