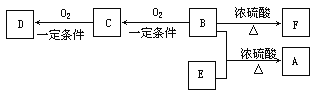
��Ŀ����
����Ŀ���ü�ʽ�廯þ����[��Ҫ�ɷ�ΪMg(OH)Br�������������л��ܼ����߷��ӻ�����]����C2H5Br(������ˮ���ܶ�Ϊ1.46g/cm3)��MgCl2��6H2O���ȿɼ�����Ⱦ�ֿɵû���ԭ�ϣ��йع���������ͼ��ʾ��

�ش�����������
��1����ʽ�廯þ��������ʱ��1molMg(OH)Br����0.5mol/L���������Ϊ_______________��
��2����������Ϊ_______________(��������)������������ʱ����IJ�������Ϊ_______________��
��3������ʱ����ҺԤ����60��ʱ��ʼͨ��������һ��ʱ�����Һ���¶Ȼ���Ȼ���ߵ�100�棬˵�����巴ӦΪ_______________(����ȡ������ȡ�)��Ӧ�������Һ���ѹ��������Ũ����Ŀ����_______________��
��4����Ũ������£��ϳ��������Ʒ�Ļ�ѧ����ʽΪ_______________��
��5������������IJ���Ϊˮϴ��Na2SO3��Һϴ�ӡ�ˮϴ���������Na2SO3��Һϴ�ӵ�Ŀ����_______________��
��6���ö��Ե缫��һ�������µ��MgCl2��Һ����ȡ���������þ���õ�ⷴӦ�Ļ�ѧ����ʽΪ_______________��
���𰸡�
��1��2L
��2�����ˡ���Һ����Һ©�����ձ�
��3�����ȣ�����Mg2+��ˮ��
��4��6C2H5OH+3Br2+S![]() 6C2H5Br+H2SO4+2H2O
6C2H5Br+H2SO4+2H2O
��5����ȥ���������ܽ��Br2
��6��6MgCl2+6H2O![]() Mg(ClO3)2+6H2��
Mg(ClO3)2+6H2��
��������
�����������1����ʽ�廯þ��ֻ��OH-�����ᷴӦ��1molMg(OH)Br����1molHCl�����������Ϊ![]() =2L���ʴ�Ϊ��2L��
=2L���ʴ�Ϊ��2L��
��2����ʽ�廯þ�����е�Mg(OH)Br�������ܽ����߷��ӻ����������ܽ���Ҫ���˳�ȥ�������������л��ܼ���Ҫ��Һ�����ȥ�����ɵ������鲻����ˮ��Ӧ��ͨ����Һ�ķ������룬��Һ��������IJ��������з�Һ©�����ձ����ʴ�Ϊ�����ˡ���Һ����Һ©�����ձ���
��3����Һ60��ʱ��ʼͨ��������һ��ʱ�����Һ���¶Ȼ���Ȼ���ߵ�100����˵�����巴Ӧ���ȷ�Ӧ���Ȼ�þˮ�����ɵ��Ȼ������ӷ��������¶�Խ�ߣ�ˮ��̶�Խ�������ѹ���������Խ��������¶�����Mg2+��ˮ�⣬�ʴ�Ϊ�����ȣ�����Mg2+��ˮ�⣻
��4����Ũ������£��ϳ��������Ʒ�Ļ�ѧ����ʽΪ6C2H5OH+3Br2+S![]() 6C2H5Br+H2SO4+2H2O���ʴ�Ϊ��6C2H5OH+3Br2+S
6C2H5Br+H2SO4+2H2O���ʴ�Ϊ��6C2H5OH+3Br2+S![]() 6C2H5Br+H2SO4+2H2O��
6C2H5Br+H2SO4+2H2O��
��5��Na2SO3��Һ���л�ԭ�ԣ��ܹ��������巢��������ԭ��Ӧ���ʴ�Ϊ����ȥ���������ܽ��Br2��
��6���ö��Ե缫��һ�������µ��MgCl2��Һ����ȡ���������þ����Ԫ�صĻ��ϼ����ߣ���������е��������������ϵõ�������������������Ӧ�Ļ�ѧ����ʽΪ6MgCl2+6H2O![]() Mg(ClO3)2+6H2�����ʴ�Ϊ��6MgCl2+6H2O
Mg(ClO3)2+6H2�����ʴ�Ϊ��6MgCl2+6H2O![]() Mg(ClO3)2+6H2����
Mg(ClO3)2+6H2����

����Ŀ��(NH4)2Fe(SO4)2��6H2O(Ī���Σ�dz��ɫ��ʽ��392)�ڶ��������г������궨������ء��ظ���ص���Һ�ı����ʣ���������ѧ�Լ���ҽҩ�Լ�����ұ�𡢵�Ƶȡ�
�ش�����������
��1��Ī�����ڿ����б����������ȶ���������¶���ڿ�����Ҳ�����������Ī�����Ƿ���ʵ��Լ���_______________��
��2��ȷ��ȡmg������Ī���Σ�����ƿ�м���20mLˮ����ܽ⣬��ij����K2Cr2O7��Һ�ζ����յ㡣�ظ�����3�Σ�����й�����������
ʵ����� | ��ʼ����/mL | �յ����/mL |
I | 2.50 | 22.58 |
�� | 1.00 | 23.12 |
�� | 0.00 | 19.92 |
��K2Cr2O7��ҺӦ�÷���______________ʽ�ζ����С�
��д���ζ������з�Ӧ�����ӷ���ʽ��______________��
������K2Cr2O7��Һ�����ʵ���Ũ��Ϊ______________mol/L(�ú�M�Ĵ���ʽ��ʾ)��
��3��ij������ͨ��ʵ�����Ī���ξ������ʱ�ķֽ���
����ͬѧ����������ֽ���������N2��Fe2O3��SO3��H2O�������ʡ����Ƿ�ͬ�Ⲣ˵��������______________��
����ͬѧ�������ͼװ�ã�����Aװ���еĹ����Ϊ����ɫ�����������к���______________��Cװ���к�ɫ��ȥ��˵����������к���______________��

Cװ�ú�Ӧ����β������װ��D��D��ʢ�е��Լ�������______________(дһ�ּ���)��
����ͬѧ����������װ��֤���ֽ�����к��а���.ֻ�����B��C�е��Լ����ɣ����������Լ�ΪB______________��C______________��
����ͬѧ��ΪĪ���ηֽ���ܻ�����N2��SO3���������װ����ѡ���Ҫ��װ�ü���֤��������ȷ������˳�������������A��______________��