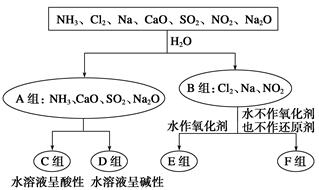
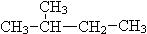��Ŀ����
FeCl3��һ����;�ȽϹ㷺���Ρ�
��1��ӡˢ��·�����ɸ߷��Ӳ��Ϻ�ͭ�����϶��ɡ�����ӡˢ��·ʱ��Ҫ��FeCl3��Һ��Ϊ����ʴҺ��������CuCl2��FeCl2����д����Ӧ�����ӷ���ʽ ��
��2��FeCl3����Ȼˮ�п����������������壬�����ˮ����������ٶȸ������Σ����������ȣ����dz�����ˮ����ҵ��ˮ�����ĸ�Ч��������ʵ������ȡ������������ķ����� ������ĸ��ţ���
| A�������͵�FeCl3��Һ�����ˮ�У������ػ�ɫҺ�弴�ɡ� |
| B����FeCl3��Һ�м���������NaOH��Һ |
| C�������͵�FeCl3��Һ�����ˮ����������������ɺ��ɫҺ�� |
| D�������͵�FeCl3��Һ�����ˮ����������������ɺ��ɫ���� |
��3��FeCl3��ʹʪ��ĵ��۵⻯����ֽ��������ѧ����ʽ���£�2FeCl3�� 2KI �� 2FeCl2�� I2��2KI������ʽ����˫���ŷ�����÷�Ӧ����ת�Ƶķ������Ŀ����Ӧ�����Һ�м���CCl4��Һ�������ú�ᷢ���²�Һ�����ɫΪ ɫ���ٽ����Һ���� �����������ƣ��У�������Һ����롣
��1��2Fe3+��Cu ��2Fe2+��Cu2+��2�֣�
��2��C��2�֣������ЧӦ��1�֣�
��3��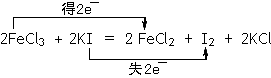 ��3�֣�
��3�֣�
��ɫ�����Ϻ�ɫ����2�֣� ��Һ©����2�֣�
���������������1��Cu��Fe3+��ԭΪFe2+����ƽ�ɵ����ӷ���ʽ��2Fe3+��Cu ��2Fe2+��Cu2+��
��2������FeCl3��ˮ�ⷴӦ����Һ���Ϊ���ɫʱ���õ�Fe(OH)3���壬��C����ȷ��A����ΪFeCl3��B���D��õ�Fe(OH)3�����������������⣻������ж����ЧӦ���ʿ��ö����ЧӦ���齺�塣
��3��FeԪ����+3�۽�Ϊ+2�ۣ���2FeCl3ת��Ϊ2FeCl2����2e?��IԪ����-1������Ϊ0�ۣ�2KIת��ΪI2��ʧ2e?��������˫���ű������ת�Ƶķ������Ŀ��I2��CCl4��ҺΪ��ɫ���Ϻ�ɫ��I2��CCl4��Һ��H2O�ֲ㣬���÷�Һ©�����롣
���㣺���⿼�����ӷ���ʽ����д����˫���ű����ת�ơ�������Ʊ������ʡ���Һ����ɫ�����ʵķ��롣

��5�֣������м������ʻ�����
| A��11H��21H | B����������� | C�����Ͱ��� | D�����ʯ��ʯī��C60 |
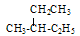 ��
��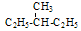 ���У�����ͬ���칹�����______������ͬλ�ص���______������ͬ�����������_______������ͬһ�����ʵ���________������ͬϵ�����_________��������ĸ��ţ�
���У�����ͬ���칹�����______������ͬλ�ص���______������ͬ�����������_______������ͬһ�����ʵ���________������ͬϵ�����_________��������ĸ��ţ� �������ӷ���ʽ��ȷ����
| A��0��01mol/L NH4Al��SO4��2��Һ��0��02 mol/LBa��OH��2��Һ�������ϲ��������� NH4++Al3++2SO42?+2Ba2++4OH?=2BaSO4��+ Al��OH��3��+NH3��H2O |
| B��NH4HCO3��Һ������Ba��OH��2��Һ��ϣ�HCO3����Ba2����OH����BaCO3����H2O |
| C����NaHCO3��Һ�м�������ij���ʯ��ˮ�����ְ�ɫ������ 2HCO3-+Ca2++2OH-=CaCO3��+CO32-+2H2O |
| D����FeCl3��Һ�м���Na2S��Һ����������2Fe3++3S2-+6H2O=2Fe��OH��3��+3H2S�� |
�����£����и������ӿ��ܴ���������� (����)
| A��pH��7����Һ��Fe3����NH4����Cl����NO3�� |
| B��pH��2����Һ��Fe2����Al3����Cl����NO3�� |
| C����ʹ�����Ի�ɫ����Һ��Cl����CO32����K����AlO2�� |
| D��������Ӧ����������������Һ��[Ag(NH3)2]����Na����CH3COO����NH4�� |
���и��������У��ܴ����������γ���ɫ����Һ����
| A��Mg2����H����C1����OH�� | B��Na����Ba2����C1����NO3�� |
| C��Na����H����Cl����CO32�� | D��K����Cu2����NO3����SO42�� |
�����£����и���������ָ����Һ��һ���ܴ����������
| A����pH=1����Һ�У�K����Na����SO42�C��HCO3- |
B����0.1 mol��L��1 Na2CO3��Һ�У�Al3����K����NO ��SO42�C ��SO42�C |
C����0.1 mol��L��1 FeCl3��Һ�У�K����NH ��I����SCN�� ��I����SCN�� |
D����c( H��)/c(OH��)��10��12����Һ�У�K����Na����ClO����NO |
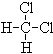 ��
��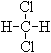 ��
��