��Ŀ����
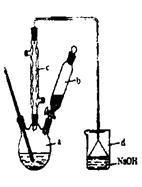
ijѧ���������ʵ��װ�����������볱ʪ����ʯ�ҷ�Ӧ��ȡ����Ư�ۣ�����һ�����ȷ�Ӧ�����ݴ˻ش��������⣺
��1��A������������ ����ʢ�Լ��� ��
��2��Ư�۽���U���в������仯ѧ��Ӧ����ʽ�� ��
��3����ͬѧ������������Ƥ���ڵIJ����ܿ�Ӧ����������ԭ���� ��
��4����ʵ��������Ca(ClO)2����̫�͡����������������Ϸ�����Ҫԭ������U���д�����������Ӧ���¶Ƚϸ�ʱ��������ʯ�ҷ�Ӧ������Ca(ClO3)2,Ϊ����˸���Ӧ�ķ������ɲ�ȡ�Ĵ�ʩ�� ��

��1��A������������ ����ʢ�Լ��� ��
��2��Ư�۽���U���в������仯ѧ��Ӧ����ʽ�� ��
��3����ͬѧ������������Ƥ���ڵIJ����ܿ�Ӧ����������ԭ���� ��
��4����ʵ��������Ca(ClO)2����̫�͡����������������Ϸ�����Ҫԭ������U���д�����������Ӧ���¶Ƚϸ�ʱ��������ʯ�ҷ�Ӧ������Ca(ClO3)2,Ϊ����˸���Ӧ�ķ������ɲ�ȡ�Ĵ�ʩ�� ��
��1����Һ©�� Ũ���� ��2��2Cl2+2Ca(OH)2=Ca(ClO)2+2H2O
��3��Cl2��ʴ��Ƥ�� ��4����U��������ˮԡ��
��3��Cl2��ʴ��Ƥ�� ��4����U��������ˮԡ��
��1������A�Ľṹ��֪��A�Ƿ�Һ©������ʢ���Լ���Ũ���ᡣ
��2����������ʯ�ҷ�Ӧ�Ȼ��ƺʹ�������Լ�ˮ������ʽΪ
2Cl2+2Ca(OH)2=Ca(ClO)2+2H2O��
��3����������ǿ�����ԣ��ܸ�ʴ��
��4�����������֪��Ҫ���Ƹ���Ӧ�ķ���������Ҫ�����¶ȣ���˿ɽ�U��������ˮԡ�С�
��2����������ʯ�ҷ�Ӧ�Ȼ��ƺʹ�������Լ�ˮ������ʽΪ
2Cl2+2Ca(OH)2=Ca(ClO)2+2H2O��
��3����������ǿ�����ԣ��ܸ�ʴ��
��4�����������֪��Ҫ���Ƹ���Ӧ�ķ���������Ҫ�����¶ȣ���˿ɽ�U��������ˮԡ�С�

��ϰ��ϵ�д�
 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
�����Ŀ