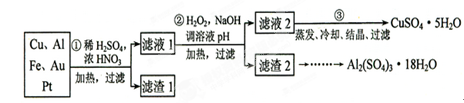
��Ŀ����
�屽��һ�ֳ��õĻ���ԭ�ϡ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
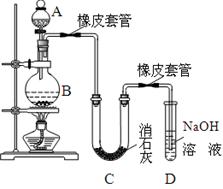
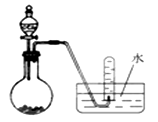
����1����a�У�װ��������ͼ��ʾ������15mL��ˮ�ı���������м���ٽ�b��4��0mLҺ���������뵽a�У���ַ�Ӧ��
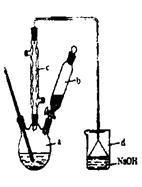
����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ8mL 10%��NaOH��Һ��10mLˮϴ�ӣ���Һ�ô��屽��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ơ����á����˼��ôֲ�Ʒ��
��1������a�������� ��
��2����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���� ��
��3������c��������������������������Ҫ������ �����ѧʽ��
��4������3�е�һ����10mLˮϴ�ӵ���Ҫ��������Ϊ ��
��5������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й��������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ����������� ��
��6��������4�Ĵֲ�Ʒ����һ�����Ƶõ�6��5mL���屽�����ʵ�����屽�IJ����� ��
����1����a�У�װ��������ͼ��ʾ������15mL��ˮ�ı���������м���ٽ�b��4��0mLҺ���������뵽a�У���ַ�Ӧ��

����2����a�м���10mLˮ��Ȼ����˳�ȥδ��Ӧ����м��
����3����Һ������10mLˮ8mL 10%��NaOH��Һ��10mLˮϴ�ӣ���Һ�ô��屽��
����4����ֳ��Ĵ��屽�м�����������ˮ�Ȼ��ơ����á����˼��ôֲ�Ʒ��
| | �� | �� | �屽 |
| �ܶ�/gcm-3 | 0��88 | 3��10 | 1��50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ�е��ܽ�� | �� | �� | �� |
��2����b�е�Һ���������뵽a�У������ܿ��ټ����ԭ���� ��
��3������c��������������������������Ҫ������ �����ѧʽ��
��4������3�е�һ����10mLˮϴ�ӵ���Ҫ��������Ϊ ��
��5������4�õ��Ĵֲ�Ʒ�л��������ʱ�����֪�����屽���й��������������ϱ�����Ҫ��һ���ᴿ�ֲ�Ʒ����������е�ʵ����������� ��
��6��������4�Ĵֲ�Ʒ����һ�����Ƶõ�6��5mL���屽�����ʵ�����屽�IJ����� ��
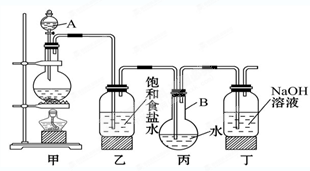
��1��������ƿ��2����ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ�����
��3��C6H6��Br2
��4������Һ���ڷ�Һ©���У�����10 mLˮ��������÷�Һ��������ϲ����ʴ��ã�
��5������6��80%
��3��C6H6��Br2
��4������Һ���ڷ�Һ©���У�����10 mLˮ��������÷�Һ��������ϲ����ʴ��ã�
��5������6��80%
��1�����������Ĺ����֪��Ӧ����������ƿ��
��2�����ڷ�Ӧ�Ƿ��ȵģ��ұ���Һ�嶼���ӷ��ģ�����Ŀ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʡ�
��3�����ݣ�2���ķ�����֪��������������C6H6��Br2��
��4���屽������ˮ�����Բ����ǽ���Һ���ڷ�Һ©���У�����10 mLˮ��������÷�Һ��������ϲ����ʴ��ã���
��5�������屽�ķе����ϴ�ͨ�����ɡ�
��6������������15��0.88��13.2g��Һ����3.1��4��12.4g�����Ը��ݷ���ʽ��֪�����ǹ����ģ����Ӧ�������屽��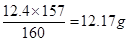 ��ʵ�������屽��6.5��1.5��9.75g�����Բ�����
��ʵ�������屽��6.5��1.5��9.75g�����Բ�����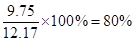
��2�����ڷ�Ӧ�Ƿ��ȵģ��ұ���Һ�嶼���ӷ��ģ�����Ŀ���Ƿ�ֹ��Ӧ�ų�����ʹC6H6��Br2�ӷ���Ӱ����ʡ�
��3�����ݣ�2���ķ�����֪��������������C6H6��Br2��
��4���屽������ˮ�����Բ����ǽ���Һ���ڷ�Һ©���У�����10 mLˮ��������÷�Һ��������ϲ����ʴ��ã���
��5�������屽�ķе����ϴ�ͨ�����ɡ�
��6������������15��0.88��13.2g��Һ����3.1��4��12.4g�����Ը��ݷ���ʽ��֪�����ǹ����ģ����Ӧ�������屽��
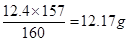 ��ʵ�������屽��6.5��1.5��9.75g�����Բ�����
��ʵ�������屽��6.5��1.5��9.75g�����Բ�����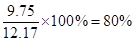

��ϰ��ϵ�д�
�����Ŀ
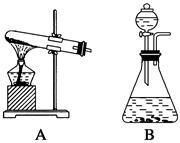
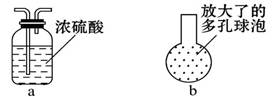
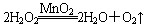 ����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��
����Ӧ��MnO2��������________����ʵ�������ô˷�Ӧ��ȡ����ʱ��Ӧѡ�õ����巢��װ����________��������鱾װ�������Եķ�����______________________________________________��